Read this article in Spanish.
Featured image: Measurement tape hanging over a forest’s soil profile near Stepanovka, Tomsk Region, Russia. The top layers show vegetation and soil rich in organic matter. Each square with an X represents each soil horizon classification.
– – –
“We [all humans] are also indigenous to the Earth. We are made of the elements of the Earth.”
Reginaldo Haslett-Marroquin, “Indigenousness”. Nov 9, 2022. YouTube. Accessed Feb 15, 2024. https://www.youtube.com/watch?v=WgTwiZL6ZGY
“Chemicals can be dangerous. […] They can […] damage trees and other plants. Chemicals can also harm the health of human beings.”
ChemHAT (Chemical Hazard and Alternatives Toolbox) []
“[…] It is possible to rehabilitate large scale ecosystems.”
Liu, John D. “Ecosystem restoration China – John D. Liu”. Sept 1, 2013. YouTube. Accessed Feb 15, 2024. https://www.youtube.com/watch?v=8WMMxvrlftA
The Universe
Where did the elements come from? How did atoms and molecules form into being? The answers to these fundamental questions lie in the origins of the universe. How did we figure it out?

~1610-1632.
Wikimedia Commons [1a].

1609.
Wikimedia Commons [2a].
Stargazing is nothing new. Humans around the world looked up at the night sky and made profound discoveries, some only with the human eye. Archeoastronomic evidence shows ancient people, as far back as 3000 BC, built their cities oriented towards to the seasonal positions of celestial objects.
In the early 1600s AD, Galileo improved Lippershey’s perspicillium and gave it to the City of Venice, then owner of the University of Padua, to monitor the Mediterranean Sea for raider ships [1]. In his spare time, he looked up towards the moon and the planets. The competition between maritime cultures created the conditions for long-distance observation technology to develop, which would later be used to discover the secrets of the Universe.
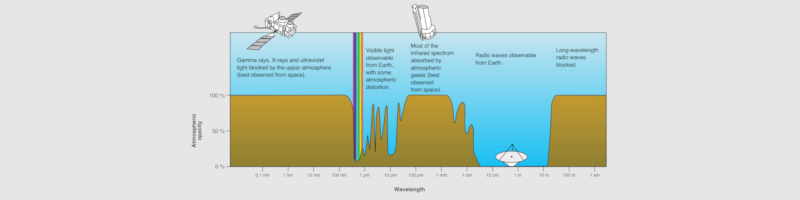
X-axis: Wavelength (0.1nm, 1nm, 10nm, 100nm, 1 µm, 10µm, 100µm, 1mm, 1cm, 10 cm, 1m, 10m, 100m, 1km)
Y-axis: Atmospheric opacity (0%, 50%, 100%)
Wikimedia Commons [3a].

Wikimedia Commons [4a].

European Space Agency (ESA) rocket in launch position (right).
Wikimedia Commons [5a].

The two colors show differences in the frequency of electromagnetic microwave signals.
Lower frequencies show the expanison of the Universe due to the Doppler Effect. As objects travel away, emitted frequencies distort and become lower, like when the tone of an ambulance siren deepens as it drives away from the listener.
Rate of expansion responsible for signal distortion is known as the Hubble Constant.
Wikimedia Commons [6a].
In the 1930s, scientists interested in radio and signals technology observed radio emissions from space. By 1937, the first antennae to observe the cosmos is built, a radio telescope [2][3][4]. When the speed of light had been taken into account, these observations suggested the universe has expanded for billions of years (1,000,000,000). The reversal of this trend suggests the universe was once highly compacted. Because of the behavior of atoms, discovered through scientific experimentation of radioactive materials, the universe is hypothesized to have started from an unimaginably dense single point. The supporting evidence and hypothesis is called the Big Bang theory.
For the last 10,000 years (Neolithic), humans observed nature and told allegories to explain reality. Institutions were created to worship these observations and other myths, accruing prestige and resources, which, for some, created confusion about the singularity of reality, what forces controlled it, and where it came from. Creation narratives passed down each generation orally, and were preserved. Some ideas have been violently enforced, or used to encourage sanctioned violence. From the social conflicts of this era arose one of the most influential creation narratives: a world created in 7 discrete time periods by a singular omnipotent force [5]. Again, today, we also observe nature and attempt a more systematic approach to evaluate it. In addition to the advantages of the scientific method, people today have the freedom to live close to ancient texts, their faith, and wisdom.
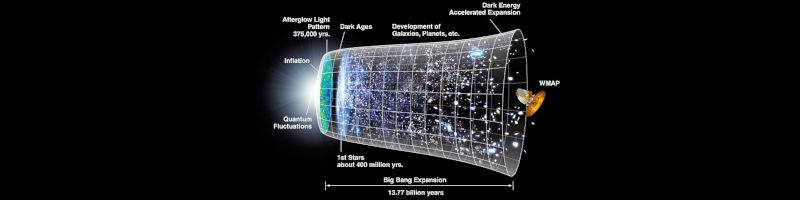
derived from Wilkinson Microwave Anisotropy Probe (WMAP) data.
Big Bang expansion (13.77 billion years): Quantum Fluctuations, Inflation, Afterglow Light Pattern (375,000 years), Dark Ages, 1st star (400 million years), Development of Galaxies, Planets, etc., Dark Energy and Accelerated Expansion.
NASA.
Wikimedia Commons [7a].
Based on mathematical calculations and other methods, scientists can determine the age of the universe: 13,800,000,000 (13.8 billion) years old [6] . Other calculations and observations suggest the universe is older than this. Scientists hypothesize the universe was so hot and dense, atoms could not form [7].
Comparable to the formation of planets, as the universe cooled, particles began to form. Hydrogen (H), an atom typically with a nucleus of a single neutron and a single proton, formed when an electron (e–) orbits stably. After the first atoms formed, photons could emit after the change of the electron position around the nucleus. More elements would be created in the intense heat and density of the universe through a process called fusion, the opposite of fission.

1150 AD.
Macrobius. Commentarii in Somnium Scipionis (Commentary on Scipio’s Dream).
Southern France.
Right: A depiction of a lunar eclipse.
Text reads: The shadow of the Earth.
1540 AD.
Petrus Apianus. Astronomicum Caesareum (The Emperor’s Astronomy).
Bavaria.
Wikimedia Commons [8a].
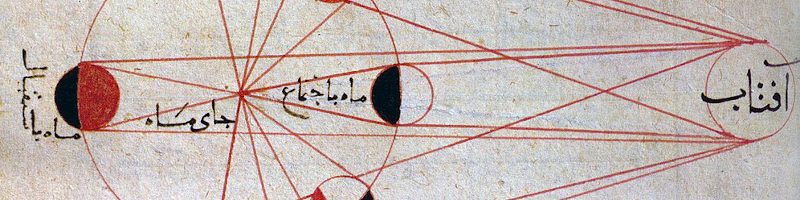
The New Moon phase (right), when sunlight is on the opposite side, can sometimes be confused with a lunar eclipse.
A moon phase is a result of us seeing one lit and one dark side.
A lunar eclipse is a momentary darkness from the Earth’s shadow.
~1000AD.
al-Biruni ﴾البيروني﴿ Kitab al-Tafhim or Book of Understanding ﴾كتاب التفهيم﴿.
Wikimedia Commons [9a].

1240-1260AD.
Joannes de Sacro Bosco, De Sphaera (Of the Sphere).
Paris, France (?).
Wikimedia Commons [10a].
Scientists have been studying stars as long as they have been able to. Miraculously enough, our star, the Sun, is located at almost the perfect distance between the Moon and the Earth for it to be completely occulted during a lunar eclipse. This feature allowed astronomers to observe the surface of the Sun and determine it was unlike a planet, but a fiery gaseous object with frequent energetic emissions [8].

1858.
Comissão astronômica de 1858 do Dom Pedro II.
Paranaguá, Brazil.
Wikimedia Commons [11a].

#CME: CME0150, classified a C8 solar flare on SpaceWeather.com archive.
Aug 31, 2012. NASA. [9][10]
Wikimedia Commons [12a].

Wikimedia Commons [13a].

Fission (right) – The fission of radioactive Uranium produces two isotopes of Barium (Ba) and Krypton (Kr).
Wikimedia Commons [14a].
Fusion and fission also occur in stars. The synthesis of new atomic nuclei from universal and stellar processes created the conditions for new atoms to form [11]. When atoms bond together they form molecules (ex: CO2, H2O, HCl, NH4, etc…). Some of the smaller atoms do not sustainably exist alone and form diatomic molecules (ex: H2, N2, O2, etc…).
Around the universe, the life and death of stars created new elements, which formed into cooler denser objects. In our solar system, 3 of the 8 planets have hard or viscous composition. These materials are heated by the Sun’s radiation. The Earth, due to a hypothesized collision with another planet, has a larger molten core, in relation to its overall mass, than any other planet. This molten core rich in Iron (Fe) generates a magnetic field, which protects our planet from most of the emissions of the Sun, allowing life to thrive.
Earth
Wikimedia Commons [15a].

Wikimedia Commons [16a].
Of the many unique characteristics of our planet, one key feature distinguishes it: water (H2O). Water is a molecule composed of three bonded atoms, two H atoms and one Oxygen atom. This simple configuration gives it unique characteristics, which help it cling to one another and carry a charge when mixed with other ionic molecules. The heat capacity of water allowed our planet to cool by absorbing heat energy after periods of cooling and heating of our planet due to volcanic eruptions. Together with the gravitational pull of the moon, water eroded the surface of our planet and formed soils.

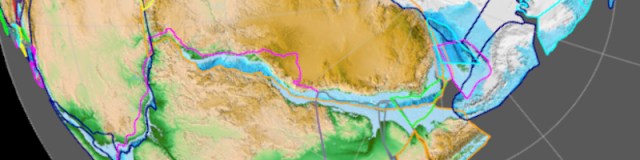
Left: Map of bedrock types and tectonic faults in South West Peninsula, UK.
Right: Diagram of soil horizon classifications used by the United States Department of Agriculture (USDA).
Credit: USDA & OpenStax Biology.
Wikimedia Commons [17a].

Green labels show the destination of magma, ocean sediments and remolten crust:
Igneous rock, Sedimentary rock, Metamorphic rock.
Credit: Syavula Education & Salt Lake Community College [18a].
The soil’s parent material is the bedrock, which is weathered and transported by wind, water, and tectonic movements to form soil. The process is so old, some of our soils today are formed from the parent material created by compressed eroded parent material. Sedimentary rocks compress weathered materials into new parent material. Sand in the ancient oceans was compressed into sandstone, which later was eroded into the soil layer above it. Limestone is also formed in a similar process but made stronger by the bonds of Calcium.
Tectonic plates or large pieces of the Earth’s crust crush along, separate, and push against each other. Even within tectonic plates there are other minor tectonic faults.
Wikimedia Commons [19a].

Once the bottom the ocean, the land shifted upwards.
Analandia, Brazil.
Wikimedia Commons [20a].
OpenStreetMap
Ancient oceans were filled with dissolved minerals, precipitated minerals and broken down minerals in sand. Some of these materials come from weathered crystals like quartz formed slowly in extreme temperatures.

Because of plate tectonics, warm tropical oceans dried and moved north.
Above these layers of ancient ocean floor, layers of soil developed from loess, a glacial dusty silt which was blown by the wind and deposited to form a new soil over the bedrock.
Byer Sandstone, Newark, Ohio
Wikimedia Commons [21a].
Open Street Map
Because of plate tectonics, masses of land move and shift. The bottom of these oceans and their weathered materials have been lifted up and are now the bedrock of new soils, and other more recent weathered materials.
Glaciers fall and push themselves, grinding rocks into dust, pushing boulders, and melt to form rivers, which move more weathered material.
Left: Glacial Maximum, Central Europe
Right: soil covered Mittivak Glacier, Greenland
Wikimedia Commons [22a].
Open Street Map
Organic rich layer near top of loess layer due to thousands of years of animal, plant, and microorganismal debris.
Tyszowce, Poland.
Wikimedia Commons [23a].
Open Street Map
Not only has water influenced the soils of our planet, but also fire. Combustion is a unique chemical reaction. In order for fire to occur, there needs to be enough Oxygen (O2) and enough fuel. Because of the chemistry of carbon-based lifeforms, plants and other organisms are the fuel source and also contributed to high oxygen-levels of our atmosphere. Without life, there would be no fire.
Fires occur naturally, and many species are dependent on fire to destroy competition, to open cones, or to encourage lush regrowth for grazing animals to eat. Despite this, fire is destructive and threatens life on Earth. Humans can create fire and developed many combustion machines, yet we still struggle to control wildfires.
As land use changes evapotranspiration patterns, increased CO2 changes global temperatures, activities near forests continue, conflict between people occur, and since historic peoples have been genocided and replaced, forest fires and other wildfires have become a significant threat to population centers. In recent years, smoke plumes have suffocated cities far away and fires have caused loss of human life.
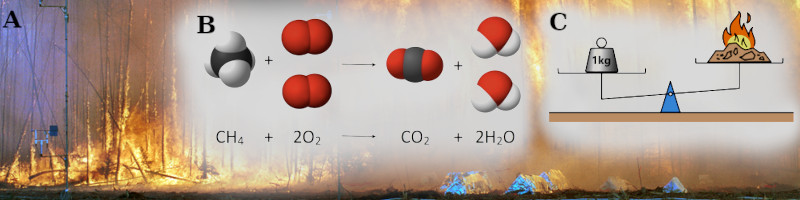
A. Research conducted in Canada by international experts uses special equipment to study how wildfires spread.
B. Combusion reaction – Under very specific conditions, organic molecules like methane (CH4) and others, will react with oxygen (O2) and release Carbon dioxide (CO2) and water (H2O). In any chemical reaction, no matter is destroyed, only transformed. Imperfect combustion of complex molecules cause smoke.
C. Conservation of Mass – Matter can not be destroyed, only transformed. Yet, if 1 kilogram of wood burns, it weighs less than a 1 kilogram weight, because matter is being transformed into gases and released into the air.
Wikimedia Commons [24a].
Chemistry

Nile crocodile (Crocodylus niloticus),
Amat Taninim Ecologic Park, Israel.
2. Kemet, one of the endonyms of ancient Egypt (hwt-ka-Ptah): four chiselled glyphs composed of crocidile skin (k), owl (m), semi-circle (t), and place marker [15]. Overlayed on the background is an image of the written Heiratic script, which could’ve traveled in scrolls to the northern port cities of Byblos, Sidon and Tyre, known to the Greeks as Phoenicia, where it could’ve re-transformed into a new chiselled alphabet and then re-adopted by the Hebrew ancient Egyptian exiles [16].
Luxor Obelisk , Paris, France.
3. Ancient Egypt excelled in manufacturing goods, creating paints, and embalming the dead. Their association with material science, may have been why Arabic scholars called the study of transforming materials, al-Khemiyah ﴾الكيمية﴿ or (that which is of) Kemet.
Compare to adjective and popular newspaper, al-Arabiyah ﴾العربية﴿.
Karnak Temple, Luxor, Egypt.
Figurine collection, Manchester, United Kingdom (UK).
4. Biblical passages in the Book of Genesis or בראשית (Bereshit), where the word חום (chum, with guttural ‘ch’) appears, meaning ‘black’, ‘burnt’ or ‘darkened’ [17][18]. This shows some linguistic similarity to the ancient Egyptian ‘Kemet’, which scholars think referred to its dark nutrient-rich silty alluvial soils.
Wikimedia Commons [25a].

Although the word ‘chemistry’ is from Africa and the Mediterranean, Chinese alchemy is one of the oldest philosophical and physical studies of material science. Most famously known for the first formulation of black powder, a chemical explosive used in fireworks and later, weapons [19].
~100AD.
Wei Boyang (魏伯陽). Zhouyi Cantong Qi (周易參同契) or The Seal of the Trinity (1.The Book of Change [I Ching, 易經], 2. Huang Lao [黄老], and 3. Chinese alchemy) [20][21].
Wikimedia Commons [26a].
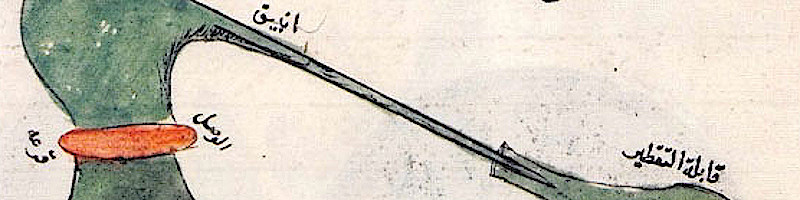
776AD.
Jabir ibn Hayyan ﴾جابر بن حيان﴿ also known as Geber,
near Iraq and Iran.
Wikimedia Commons [27a].
Far into the archeological record there’s evidence phytochemicals dyes used by humans. Humans around the world harvested soils and processed clay, harvested ore and smelted metal. Humans also used paints, adhesives, and oils. These substances came from minerals, plants, or animals.
Our ability to manage and transform materials amazes ourselves. People believed they could magically transform common metals into gold. What began as an informal learning process became infused with superstition. Modern chemistry distinguishes itself from these superstitions by adhering to the scientific method, which focuses solely on the demonstrable and repeatable.
Chemistry, the modern science, structures and classifies materials [22][23]. These advancements allow us to look up at the skies with more certainty about the behavior of electromagnetic wavelengths and the creation of new elements. It is the reason why we can describe plant life in terms of elements, atoms, molecules and reactions.
Wikimedia Commons [28a].
Chemists divide organic chemistry, chemical reactions inside living organisms, from non-organic chemistry, chemical reactions between non-living materials. However, non-living materials like Lead (Pb), Iron (Fe), and Nickel (Ni) interact with organisms in destructive ways.
Early observations, philosophical thoughts, and experiments have led to us create and manage our world. Because we know everything is made out of atoms, it looks like it only created the problem of new dangerous substances, but now we can also study the natural dangers that existed before humans.

String Theory – The vibration of subatomic waves generate particle physics behavior.
Wikimedia Commons [29a].
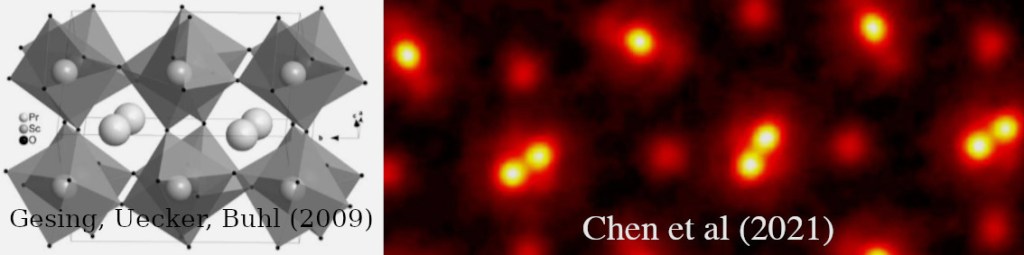
Right: A high resolution ptychographic image shows the larger atoms Praseodymium (Pr) and Scandium (Sc), yet Oxygen (O) is not visible.
(Chen et al. 2021) (Giesing, Uecker, Buhl 2009) [24][25].
Inside atoms are neutrons, protons, and electrons. Atoms in the periodic table are sorted by their atomic number or number of protons in nucleus, which correlates to its atomic weight. When ordered by this characteristic, they change periodically (same repeating pattern) in their atomic radius, which measures half the diameter of the nucleus, and correlates with the amount of electrons they can hold.
The reason why some atoms are more reactive than others are because electrons exist in pairs. When a neutral atom has an odd atomic number (number of protons = number of electrons, unless it is an ion), the atom wants to bond to other atoms to pair their electrons. When a lone pair exists, it changes the geometry of a molecule and any emergent crystal or amorphous structures. Ions are atoms with a positive or negative charge thanks to less or more electrons than the number of protons.
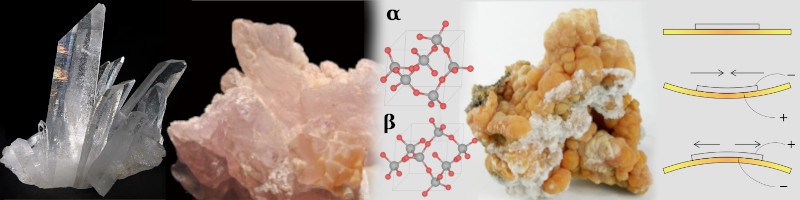
Quartz is a mix of different Silicon (Si) and Oxygen (O) molecules in crystal formation (alpha, beta structures).
Quartz can have many colors, because of other elements its crystals are mixed with. It can also form amorphous structures.
Quartz is used in handwatches as a power source. By bending a quartz crystal, piezoelectricity can be generated.
Wikimedia Commons [30a].
Molecules are bonded atoms. The word, chemical, is an ambiguous term used for any unique substance created from the reactions of atoms and molecules [26]. We tend to associate the word ‘chemical’ with synthetic or anthropogenic substances (created by humans) or dangerous substances, yet water and other common substances can also be considered chemicals.

1. Rainbow over poplar trees (Populus spp.) in Mariupol, Ukraine.
2. Sunlight – visible electromagnetic wave.
3. Raindrop (H2O and others) – Raindrop shape created in mid-air by hydrogen-bonds between H2O molecules, which is also called cohesion. Light wave changes direction because of the curvature of the raindrop.
4. Light wave inside raindrop can’t escape and reverberates like a mirror. Because of distortion from step 3, the orientation of frequencies in space changes directions.
5. The light waves which used to modulate each other through an additive synthesis to create white light, become separated in trajectory.
Wikimedia Commons [31a].
Applied Chemistry

The behavior of electromagnetic waves in response to other particles or waves can tell us information about the source material.
First observed and recorded by botanist Михаил Цвет (Mikhail Svet) around 1903. When paper with plant extract is wet, the colors travel to different distances of the water adsorbed around microscopic spaces between paper fibers [27][28].
Mikhail observed how the splotches of different colors corresponded to different pigment molecules in the plant extract. The molecular weight or volume (density) and quantity of pigment molecules could be inferred from the smears, visually.
Chromatography is now a technique used for many other sciences and industries.
Wikimedia Commons [32a].

As time went on, scientists discovered they could use different media to identify molecules. In 1944, gas was used to disperse the vaporized studied material and detect the composition of the molecule from the atomic reactions to light or other detectable electromagnetic signal [29].
Wikimedia Commons [33a].
As the methods improve to detect and survey the chemical composition of plants, their nutritional value, or their contaminants, thresholds change. Before, substances used to be measure in ppm (parts per million) and then increased to ppb (parts per billion). If we have the ability to find the smaller and smaller traces of chemicals, at what point do we decide what is significant and what is not? How can we make chemical analysis meaningful to our needs? Scientists conduct research to determine when the presence of a chemical is relevant and when it is not.
The Elements

Many cultures have recognized the world being composed of fundamental elements. Sometimes it is two dual elements (ex: Yin and Yang), three elements (ex: Salt, Mercury, and Phosphorus), four elements (ex: earth, water, air/wind, fire), five elements (ex: the four Aristotelian elements plus ‘spirit’ or something similar), and so on.
These elements were placed in graphical representations to show their relationship with one another. They were also given symbols.
Wikimedia Commons [34a].

The Periodic Table is the one of the most accurate categorizations of all known elements.
Down each column (Y-axis, red arrow) the radius of the atoms increases.
Along each row (X-axis, blue arrow) the number of protons, known as the atomic number, increases.
The elements are grouped in blocks, which correspond to the increase of energy levels (n=1,2,3…) and the probabilistic superlocation of electrons, known as orbitals (s, p, d, f).
These colors of periodic table indicate their sub-categories: Diatomic nonmetal (green), Alkaline metal (red), Alkaline earth metal (~orange), Lanthanide (pink), Actinide (dark pink), Transition metal (salmon pink), Unknown (grey), Post-transition metal (dark grey), Metalloid (brownish green), Halogen (yellow), and Noble gas (light blue).
Wikimedia Commons [36a]
The elements of the universe are a long list [30][31][32]. In the plant sciences, only a limited series is important. Thankfully, the Earth is abundant in the right kind of elements for life to thrive.
Hydrogen
At the temperature and pressures of Earth’s surface, elemental or pure Hydrogen (H) commonly exists as a gas. People used to think all gases were the same. During the times of Greeks and in many other cultures, the four primary elements were: air, fire, earth, and water. Today these are called the Aristotelian elements and all related studies Aristotelian physics. Scientists like Antoine Lavoisier, among many others, discovered there were different types of gases, not just air. When he produced hydrogen, he could light it on fire and produce water. He suggested the name ‘hydrogen’, which means ‘water producing’ in Greek []. Hydrogen atoms are not only found as gases but inside the molecules of many other substances.

Wikimedia Commons [37a].

Since Hydrogen (H) is simpler, it is often used as the model element to describe behavior of atoms.
n = 5, refers to the 5th energy level of the H atom, the outermost dark grey circle.
Wikimedia Commons [38a].

This spectral emission chart of Hydrogen is limited to the range of visible electromagnetic wavelengths (light).
Wikimedia Commons [39a].
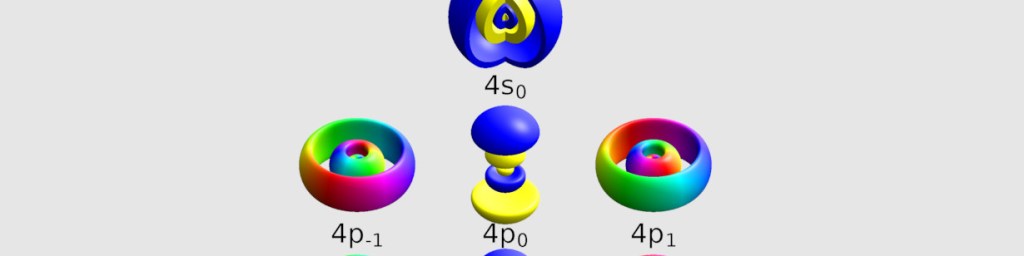
Orbitals model the predicted behavior of electrons (e–). Since electrons can exist in multiple places at the same time (superposition), until measured, they are calculated as existing within a 3-dimensional field of 90% probability. Outside of these shapes, there is 10% or less chance of finding an e–.
These colorful models are used to help see the shapes of the electron probability clouds. Greater energy levels means more electrons. These geometries are calculated with mathematical equations. Four variables known as quantum numbers correlate to physical characteristics.
Wikimedia Commons [40a].
Hydrogen plays a crucial role in biochemistry. In many ways, it is a conduit for electro-chemical activity, because of how easy it is for molecules of greater polarity to break the bonds of Hydrogen to other atoms. However, the bonds between Hydrogen atoms are so strong for their size, they also contribute to the behavior of liquids and chemical reactions.
Hydrocarbons like ethanol are created from the fermentation of plant material. These molecules have been important, because they are mixed with less abundant hydrocarbons used as fuel sources for combustion engines in cars and planes.
Carbon
The element name for Carbon (C) comes from the Greek word for charcoal. Charcoal has been produced since ancient times by burning wood under a covered pit or structure, so the wood doesn’t burn all the way. Charcoal is easier to burn and burns hotter than normal wood. Antoine Lavoisier proved diamonds were made of the same element as charcoal by measuring the weight of diamonds and Carbon dioxide gas (CO2) in various heating experiments. According to our current understanding of life on Earth, there are no living organisms without Carbon atoms in their molecular structures.
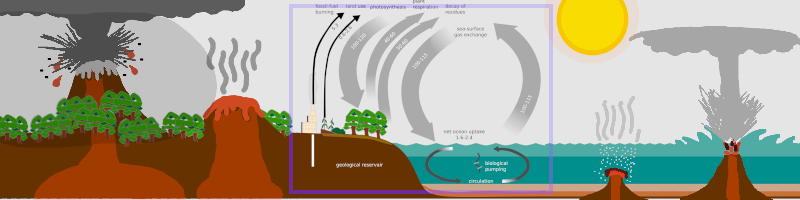
Gray and black arrows show the proportion of Carbon dioxide (CO2) intake (downward arrows: plants, gas exchange in the water, aquatic organisms) and exhaust (upward arrows: plants, plant cultivation, factories, gas exchange) .
Think outside the box:
Consider how different types of volcanoes interact with the atmosphere. All of them release CO2, which contribute to the greenhouse effect, yet many types release particles into the air that block sunlight.
Wikimedia Commons [41a].

Wikimedia Commons [42a].

Center: Humans have used coal and charcoal since early times as a black pigment in cave paintings and other arts.
Right: Charcoal is a carbon-rich resource produced by burning wood under controlled conditions. It burns at higher temperatures than normal wood, helpful for pottery (ex: flower pots), smelting and working metal (ex: garden tools), and cooking.
Wikimedia Commons [43a].

Wikimedia Commons [44a].
Carbon is one of the most important elements for plant life [36][37]. Today we often associated Carbon with the rapid production of Carbon dioxide (CO2) from the combustion of fossil fuels and their consequences on the global climate. Yet, Carbon plays an essential role for plants.
As a seed, organic molecules with Carbon skeletal structures like carbohydrates (sugars) and hydrocarbons (lipids) store enough Carbon for the plant to create its body as it establishes its first leaves and roots. Once the leaves are established, the stoma expel some water and intake CO2. This CO2 becomes the primary source of Carbon utilized in photosynthesis, which metabolizes a CO2 gas molecule into a carbon chain molecule, then into a sugar.

A cross section of a leaf from a species of Privet (Ligustrum sp.), an ornamental shrub and C3 plant (left).
A cross section of a Sugar Maple (Acer saccharum) leaf, a C4 plant (right).
Vanderbilt University [45a].
Not all plants manage this Carbon resource the same way. Botanists have recognized three major groups of plants based on their metabolic pathways, which specialize in conserving the carbon molecules, before they re-oxidize and return to the atmosphere as CO2. These metabolic pathways are C3 (3 carbon chain intermediary molecule), C4 (4 carbon chain intermediary molecule) and CAM (Crassulacean [Cacti, etc..] Acid Metabolysis) [38][39][40].
Not only do these photosynthesis pathway groups represent metabolic differences, but also physiological differences. For example, C4 plant leaves have structures around their vascular tissues called bundle sheath cells. Within these groups, there are also more in-depth distinctions. In all, Carbon metabolism is a complex process, which plants have adapted to do more creatively than many other organisms.
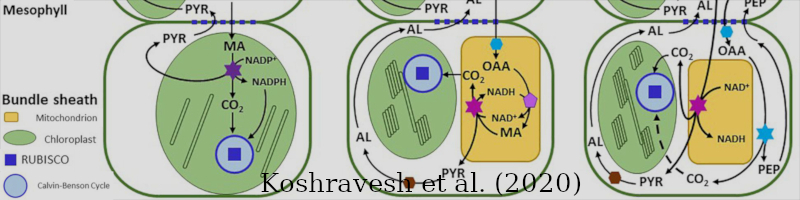
RuBisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase) is an enzyme present in C3, C4 and CAM metabolic pathways.
Koshravesh et al. 2020 [46a].

Cross section of water lily leaf shows air pockets in mesophyll, which allows them to float.
Some water lilies metabolize Carbon similar to Cacti.
Scientists name this subcategory of CAM pathways as Submerged Aquatic Metabolism (SAM) [41].
Michael W. Clayton.
UW-Madison Digital Collections (UWDC) [47a].
Scientists have been observing the greening of forest leaves due to the greater availability of CO2 in our atmosphere. While this may sound like CO2 is not bad, it contributes to excessive warming of our planet. Together with the land use changes, the warming of the atmosphere by trapped solar heat radiation causes water to evaporate and plants to stress, which causes desertification. This affects the climates of many regions since they rely on evapotranspiration from plants, and other wind and ocean currents [42].

Cellular structures preserved in carbon-rich inorganic material.
UW-Madison Botany 305: Plant Morphology and Evolution, Mar 2022.
Photographed by Gustavo Meneses (iPhone SE).
Carbon is so important to the structure of plants, paleobotanists and archeologists can determine a plant species from the structure of charcoal (woody plants burnt by humans in oxygen deprived conditions) found in ancient remains. Paleobotanists and archeologists also can study much older fossils of plants in coal (compressed swampy plants and other biota). This is how scientists study plant evolution and morphology. By comparing carbon structures in these inert materials, scientists can compare changes in the morphology of plants and determine how leaves, vascular tissues, spores and species diversity changed over million of years, a countless number of generations.
Oxygen
The elemental form of Oxygen (O) in the temperatures and pressures of the surface of the Earth is a gas. Scientists proved Oxygen is involved in the reactions which cause fire (combustion) and those which cause rust (oxidation). Early chemists knew both rust and fire were similar chemical reactions, but thought it was produced by a hypothetical element called Phlogiston, which was never discovered and later disproved.

Right: Liquid oxygen container, used as rocket and jet fuel.
Wikimedia Commons [48a].
Because we and all the other animals need oxygen to breathe, we tend to portray Oxygen as a good element. Yet, chemicals aren’t good or bad. They are reactive or unreactive. Some reactions can be bad for certain system functions, and some can be good for system functions. Oxygen is needed to carry out important reactions in our bodies. Many reactions in plants need Oxygen as well. Yet, if plants have too much Oxygen, destructive reactions can occur and will cause the plants to accumulate harmful metabolites it can’t properly metabolize.
Because Oxygen is so reactive, it likes to bond with Hydrogen. These bonds are so strong, it takes energy for us to break them manually with an electrical current. Many countries are experimenting with transforming the force of wind to generate an electric current and break water molecules (H2O) into a storable Hydrogen gas fuel through the process of electrolysis.
Plants also break the bonds between Hydrogen and Oxygen in water when they photosynthesize. Plants use the Hydrogen atoms from water and the Oxygen atoms from metabolized CO2 to create sugars [Ca(H2O)b]. The unbroken molecules of water are evapotranspirated. The Oxygen atoms from water become the diatomic Oxygen gas (O2) we breathe.

Wikimedia Commons [49a].

The light red or pink background is water. The small circles are particles in solution called solutes. The concentration of solutes on right side is higher, H2O molecules want to distribute themselves around the solute molecules.
Since volume of liquid on right side changes, it creates osmotic pressure.
2. Osmosis in real life – Salt on the mesocarp of a sliced eggplant (Solanum melongena fruit) causes H2O inside cells to leave towards the thin high solute concentrated solution on the exposed layer.
3. Diagram shows how water enters roots. Osmosis causes water to enter outer cell, in this case a root hair cell. Blue line shows path of solution (water & nutrients) inside cells, called symplastic pathway. Red line shows path inside cell membrane, called apoplastic pathway.
4. Once solution reaches vascular tissue cells, the hydrogen bonds between H2O molecules carries the solution upwards the plant to where it exits the plant.
Wikimedia Commons [50a].

Evapotranspiration bag, survival technique.
Red arrow: Small openings in lower epidermis are created by stoma (pl. stomata), open when guard cells fill with water. CO2 in atmosphere enters here. Some plants have stomata on top of leaf and bottom, proportions vary, yet bottom normally has more.
Yellow arrow: Light waves enter palisade layer of mesophyll. Below it, spongy layer of mesophyll. AKA, palisade mesophyll, spongy mesophyll. Photosynthesis reactions occur in chloroplasts within palisade layer cells.
Blue arrow: H2O and Oxygen (O2) leave the plant in their gas state. The process of losing water has many functions for the plant. This process is called evapotranspiration. Plants lose a majority of their water in this process to keep the chain of molecules moving throughout the vascular system. Gaseous water can form mist over forests, enters atmosphere, and can change weather, and over long periods of time, change climate.
Wikimedia Commons [51a].
Water is the most important molecule for plant life, and the reason why Oxygen is so important. Water is a polar molecule and has some curious electromagnetic properties. It is the most abundant liquid at ambient temperature and is therefore the medium for which all nutrients are delivered throughout the bodies of not only plants, but all other animals.
Curiously, the English word ‘Soul’ shares a common root with the German word ‘die Seele’, which also means soul, but is known to derive from the words ‘die/der See’, which traditionally is translated into English as ‘a lake’ or ‘a body of water’. Wolf-Dieter Storl, a contemporary German naturalist, recounts this and observes how plants have souls, even though they are not as animated as those of animals.

Left: Cultivation of sugar cane (Saccarum officinarum), which can be chewed raw, cooked into a slurry called molasses, or refined into crystal sugar. Sugar cane was introduced to the Carribean from its center of origin near New Guinea in Southeast Asia around the 1500’s. The sugar industry in the Americas was created by slaves.
Photo: Oct 7th, 1929. Yogyakarta, Island of Java, Indonesia.
Middle: Cultivation of sugar beets (Beta vulgaris Altissima Group). Sugar beet cultivation in Europe represented a cheaper and ethical alternative to sugar cane.
Photo: May, 1943. near Stockton, California, USA.
Right: Cultivation of sorghum (Sorghum spp.), a crop native to Africa. Sorghum, along with many other crops, can be processed into a sugary dark syrup, similar to molasses in appearance.
Photo: Apr 5, 2022. Rwanda.
Wikimedia Commons [52a].
Nutrients
Of all the elements, there are three top limiting nutrients, which are considered the macronutrients of plants: Nitrogen (N), Phosphorus (P), and Potassium (K).
A macronutrient is an element, usually found in a functional molecular form, which is required by a plant at greater quantities [45]. If there isn’t enough of the limiting nutrients, then the plant can’t grow and incorporate the other nutrients. In contrast, micronutrients are elements which are required at quantities and/or rates, which are easily met.

Trees that grow in nutrient deprived conditions, such as rocky cliffs, rocky beaches, and rock crevices, can be a thousand years old but look similar to younger thinner trees [46].
Without nutrients, plant growth will be stunted. Most of these examples are isolated specimens. In these conditions, communities of plants can’t thrive [47].
Wikimedia Commons [53a].

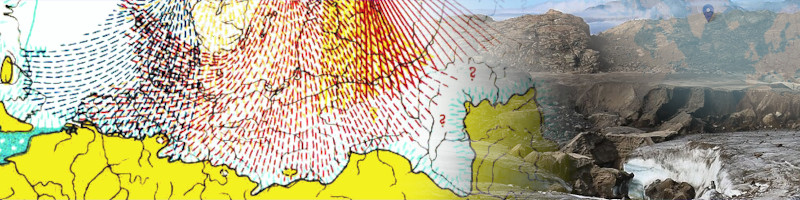
The illustration on the right shows the mechanics and process of podzolization, which creates the podzol or albic layer typical of the order Spodosol and found within different orders in USDA soil taxonomy (ST) [48].
There is no consensus in soil taxonomy. Various countries have their own system of classification, which can be confusing [49][50][51][52].
Wikimedia Commons [54a].
Cultivation & Soils
In any system of cultivation, when nutrients are introduced to encourage growth or another plant function, they are often called fertilizers. Fertilizers and all the other added substances are called inputs.

1. Soil – Layers of weathered organic and inorganic material outside.
2. Dirt – Commonly used to describe soil. Soil scientists, like Doug Soldat, prefer the term soil and say, “Dirt is on the shirt.”, to explain how dirt is negatively perceived by society.
3. Sphagnum moss (Sphagnum spp.) – A genus of bryophytes.
4. Sphagnum peat moss (soil amendment) – Harvested moss from ancient deposits of Sphagnum and other organic materials.
5. Potting mix – Most often a mix of soil-less media (Sphagnum, coconut coir, etc…) and other inorganic porous materials (perlite, vermiculite, etc…).
Wikimedia Commons [55a].
Usually, plants are able to find micronutrients in outdoor environments. In indoor environments like greenhouses, cultivators find it easier to use soil-less media (ex: Spagnum moss) instead of the pathogen or pest filled soils (ex: insects, fungi, bacteria). Sometimes, people will pasteurize soils by heating them (160°F or 71°C) and killing any germs [53]. Many prefer soil-less media because it less heavy and drains easily, which makes it easier to avoid soil compaction, overwatering, and root rot.

1. Hydroponic systems – Hydroponic systems use a substrate for water to flow through. In this case, this system probably uses plastic covered rockwool (molten rock spun into fiber). Other systems have openings where roots can grow directly in water or mist.
2. Plastic mulch film and soil liner – Plastic is used by some cultivators as a method to cover the soil to prevent weeds on exposed soil, or to cover below or inbetween soils to prevent plant roots and stems to grow into the cultivation bed.
3. Flats and cell-packs – Moldable and separatable containers can grow plants to a desired size, or are used to sell plants.
4. Plant propagation – Different media (sand, perlite, vermiculite, agar, etc…) are used to propagate plants from stem-cuttings or some other tissue.
Wikimedia Commons [56a].
The top ingredient in soil-less media, Sphagnum moss, is criticized because it is harvested from ancient sensitive ecosystems, where it doesn’t grow fast enough to replace. Plant breeders are researching how to cultivate Sphagnum moss.
Sphagnum moss lacks macro- and micronutrients. Within indoor cultivation systems, micronutrient profiles are important to consider if a high-quality healthy product is desired. Growing guides provide research-based advice on what quantities of micronutrients are needed for each crop. Independent empirical research could determine if growing guides are influenced by economical industry expectations.
Plant nutrients aren’t only in soils. Plant microbiome plays a role in distributing nutrients throughout various organs in the plant, besides the roots. Nutrient allocation is important to cultivation. Some growers will spray fruit with Calcium (Ca) fertilizers to ensure the nutrient travels to the fruit before it goes to new vegetative growth, since Ca-deficiencies in the fruit correlate with pest damage.
The top three macronutrients of plants are Nitrogen (N), Phosphorus (P) and Potassium (K). This trio is often referred to as NPK. Fertilizers, organic or synthetic, are measured by an NPK ratio. The NPK ratio (N:P:K) represents the percent of the weight available nutrient (molecular weight) within the weight of the fertilizer product, except for N. Nitrogen is measured by percent of elemental weight (atomic mass) within weight of the fertilizer product.
Fertilizers, organic and synthetic, but moreso synthetic because of the concentration of nutrient, are capable of damaging plants by changing the molarity (concentration of solutes in a solution) of water in the soil. If the molarity of soil matrix water is higher than the plant, the water in the roots moves to the soil because of osmosis. Plants do not suck up the water from the soil, but instead use osmotic chemistry of the soil and root tissues to encourage water molecules to bind to the solutes within the root cells. Membranes allow the passage of water between root cells and soil. If the chemistry of the soil changes, it can cause the osmosis to reverse. If molarity affects osmotic behavior, it is measured as osmolarity.
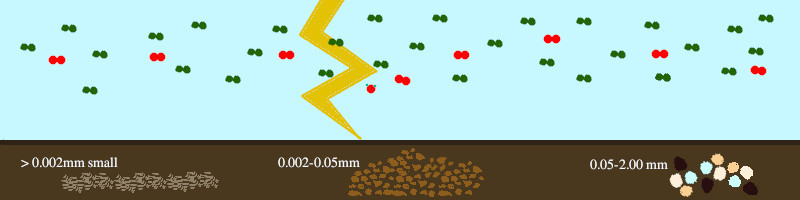
Atmospheric gases:
Diatomic molecules of Nitrogen (green) and Oxygen (red) shown in proportion. At this scale (30 nitrogen diatoms), there aren’t enough CO2 molecules to draw one [54].
Most chemists focus on a mineralogical perspective, where Nitrogen diatomic gas (N2) can only be turned into fertilizer by lightning or generated electricity.
Soil minerals:
Bottom Left: Clay (less than 0.002mm or 2µm) has layers of complex crystal structures, which can trap Iron (Fe), water(H2O), and other particles. This is why certain clays have greater volume when they’re wet, and they shrink and crack when they dry [55][56].
Middle: Silt (between 2µm and 50µm) is what we often refer to as dirt. You can feel the difference when it is wet. It does not bind together and become malleable like clay. Since it has larger particles, water can travel through silt easier than clay
Right: Sand (between 50µm to 2mm or 0.2cm) can come from many minerals, most often Silicon dioxide (SO2), and in many different colors. Dark sands are younger and indicate volcanic events. Water passes through sand the quickest. Sand’s size can cause fertilizer leakage into underground water, especially because sandy soils are preferred for root crops like potatoes (Solanum tuberosum).
PlantResearchORG (PRO).

Left: An Scanning Electron Microscope (SEM) image shows clay particles of the red soils and rocks of the Grand Canyon, known as the Supai Group. Red color is from Ferric oxide (Fe2O3) molecules located mostly on the surfaces. Water molecules can fit between layers.
Grand Canyon, USA.
Middle: SEM image of an industrial sand, produced by modern human activities in ancient sand deposits.
Illinois, USA.
Right: Microscopic image of coarse sand from a beach, caused by erosion of sea creature shells and minerals for millions of years.
French Bay,
San Salvador Island, Bahamas.
Wikimedia Commons [57a].
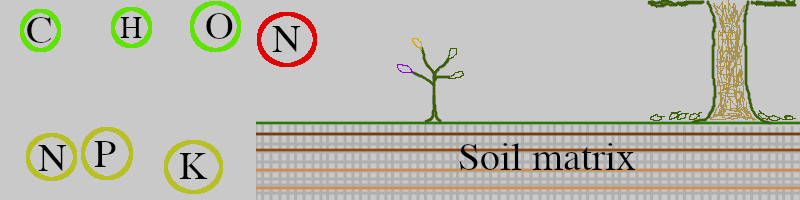
Green circles are available elements. Yellow-green circles are limiting nutrients: the plant won’t grow more or use another micro-nutrient, if there is not enough of these in their bio-available forms. Red circle shows the abundance of Nitrogen in the atmosphere, which is unavailable to the plant, yet available to microorganisms.
Soil is a microscopic labyrinth filled with water (soil solution), and composed of layers (horizons). The very bottom layer is the parent material or bedrock. Unless there was an event by air, water or organisms that displaced material from somewhere else, the second to top layers are weathered parent material. All of these forces contribute to the chemical profile of soil types.
Soils are organized into groups called soil orders. Countries share or have their own methods of classification. One USDA soil order to remember are Mollisols, and within them is the suborder, Chernozem (Чернозём), classified by first modern soil scientist, considered the founder of the science, Василий Докучаев (Vasily Dokuchaev) [57].
Plants’ focus on the nutrients in the top layers, and are affected by the mineral composition of the bottom layers. The very top layer is the duff layer, where leaves and organic materials decompose. Plant metabolites in leaf litter can control microorganismal activity.
Leaf coloration is one observable indicator of plant nutritional stress. Leaf texture can also change with stress. Other advanced methods to detect nutritional stress involve sample preparation and lab analysis.
PlantResearchOrg (PRO).
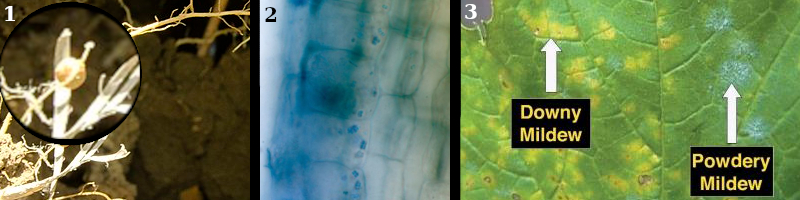
1. Microbiome – A macroscopic example in the soil: Soybean (Glycine max) nitrogen-fixing bacteria nodules.
2. Rhizosphere – All around the roots and inside the root cells, bacteria, fungi, and other critters, contribute to plant nutrient management.
Example: Micrococcus luteus inside cells of Curly Dock (Rumex crispus).
3. Phyllosphere – The surfaces of leaves are not only places where pathogens cause diseases, but also places where beneficial microorganisms protect and complete important functions, like reactions with methane (CH4).
Example: Downy Mildew of Grapes (Vitis spp.) disease caused by pathogen Plasmopara viticola, & Powdery Mildew of Grapes disease caused by pathogen Erysiphe necator.
Wikimedia Commons [58a].

2. Mulder’s Chart – Nutrients in the soil react to each other chemically. These reactions cause changes in nutrient availability. If the available nutrients of one element (ex: Ca) are abundant, the nutrients with an antagonistic relationship will be less available (ex: Mn, K, Fe, P, Mg, Zn, B). In synergistic relationships, both elements are available together.
Hortipendium. Homo agricola [59a] .
Primary Macronutrients
Nitrogen

Middle: Chilean Saltpeter – mined from ancient deposits of bird guano, source of Nitrogen-rich fertilizer. Product labels (German: 167% Nitrogen) can often be confusing or untrue. Certain countries regulate what information is presented in the fertilizer product label.
Right: Nitrogen (blue) – abundant inside protein molecules.
Wikimedia Commons [60a].
Add one proton to a C atom, and you’ll get an isotope of Nitrogen (N). Nitrogen is the most abundant element in our atmosphere, as the diatomic molecule N2. Nitrogen is one of the most important elements for plant growth, because of its existence in every protein molecule and because of the scarcity of mineralized Nitrogen, like Nitrates (NO3–) in soils. Organic Nitrogen-containing molecules like Ammonia (NH3) are much more common.
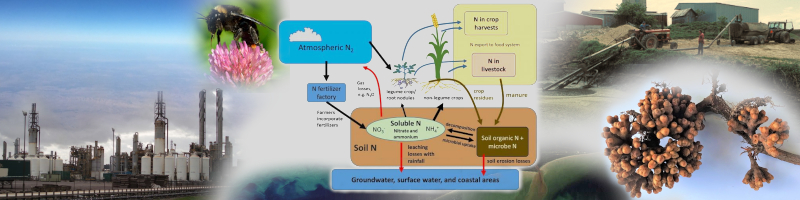
Nitrogen gas (N2) is the most abundant gas in atmosphere and is artificially turned into bioavailable salts with a lot of energy.
Leguminous plants and others have special relationship with Nitrogen-fixing bacteria that mineralize organic N sources. These bacteria live inside the cells.
Because N is the most needed macronutrient and water soluble, it contributes to a lot of environmental problems. In some places, laws restrict farmers from creating manure pools near rivers. If drinking water is contaminated with too many nitrates, it can negatively affect the fetus in pregnant women.
Originally from Penn State publication and Steve Vanek.
Wikimedia Commons [61a].
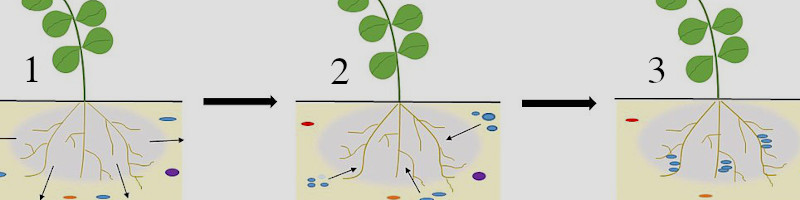
1. Plants’ roots exude biomolecules called exudates. Gray circle is area within soil matrix influenced by roots, called the rhizosphere.
2. Nitrogen-mineralizing microorganisms colonize the rhizosphere.
3. Microorganisms consume carbohydrates from plants, and microorganisms increase supply of available N. Relationship helpful for the survival of both species in stressed environments.
Wikimedia Commons [62a].
Phosphorus

Wikimedia Commons [63a].
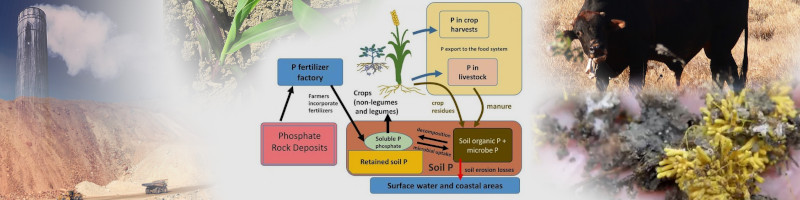
Phosphorus cycle includes artificial inputs (mining, chemical factories), natural inputs (animal waste, crop residues, microbiotic relationships, soluble P in soil solution), and outputs/unavailable sources (retained P in soil matrix/minerals, organic P, crop harvests, livestock harvest).
Originally from Penn State publication & Steven Vanek.
Wikimedia Commons [64a].
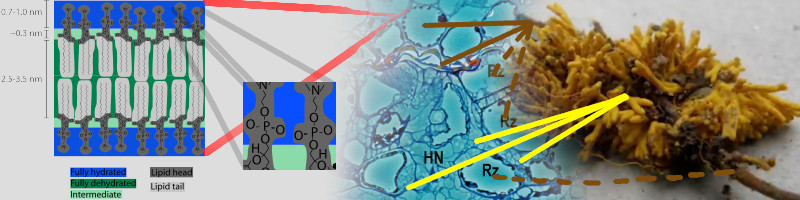
Soil microbiome interacts and provides available P to plant roots. Ectomycorrhizal (EcM) fungi colonize the epidermis of plant roots (dashed brown lines). EcM spread hyphae network called the Hartig Net (yellow lines) between the cells, except for the endodermis and interior root cells (brown lines).
90% of plants have some sort of relationship with mycorrhizal fungi. Another example, arbuscular mycorrhizal fungi (AMF).
Wikimedia Commons [65a].
The elemental form of Phosphorus (P) was discovered when chemists boiled urine until all the liquids evaporated. The residue ignited and burnt brightly, thus the element was named φωσφόρος (fosforos, light-bearer), an ancient Greek word from φῶς (fos, light) and φέρω (fero, carry).
Although less P is needed compared to N, it plays a crucial role in the formation of Adenosine triphosphate (ATP), which is the energy carrying molecule in biomolecular reactions. For this reason, it is essential in the fundamental cellular processes like DNA formation, among others. It plays a role in how plants manage abiotic stresses like drought [63].
Unlike N, P binds easily to other minerals in the soil matrix and can become unavailable. P in organic sources like urine must be diluted in water to avoid salt damage. Excessive P from artificial fertilizers, erosion, and other sources drain into and change aquatic environments by promoting algal growth. If algae become over-abundant, they die and decompose, they consume Oxygen in the water and suffocate fish, aquatic plants and other organisms, which need it for respiration. This process is called eutrophication.
Potassium

Wikimedia Commons [66a].
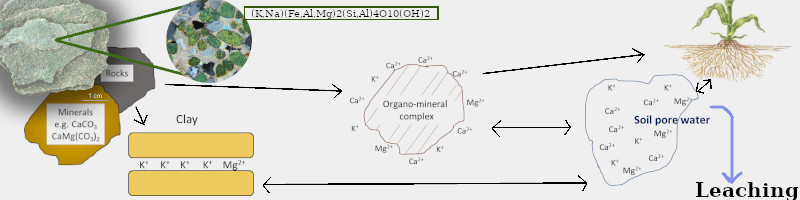
The green mineral on the left is infused with small crystals of glauconite. When mined and sold for horticultural use, it is called greensand, an organic source of K.
Originally from Joann Whalen & University of Saskatchewan publication.
Wikimedia Commons [67a].
Potassium (K) is the tricky one to remember since it is K, which stands for Kalium, from German and the out-dated English word kali, which comes from the Arabic word al-Quali ﴾القلي﴿, which means potash.
Potash has been known for thousands of years and was discovered when someone boiled the ashes from burnt wood in a pot and collected the residue [65][66]. Potash was documented by the Sumerians in ~1800BC. It is one of the oldest ingredients of soap. The elemental form was discovered when alchemists separated earths, as they had separated water into Hydrogen and Oxygen using electrolysis. Separating Potash, they produced Potassium metal with exothermic reactions when placed in water (See here).
Today, potash refers to all Potassium salts used in fertilizers, thus potash mines are places where minerals with K are extracted from the Earth and processed.
Potassium plays an important role in facilitating enzymatic activity in plant physiological processes like photosynthesis, stomatal regulation and wood formation [67][68]. It is found in many minerals, like glauconite, yet it takes a longer than expected to leach into the soil and become available. For these two reasons, K is a limiting nutrient and options for K fertilization is limited by release time [69][70].
Secondary Macronutrients
Magnesium
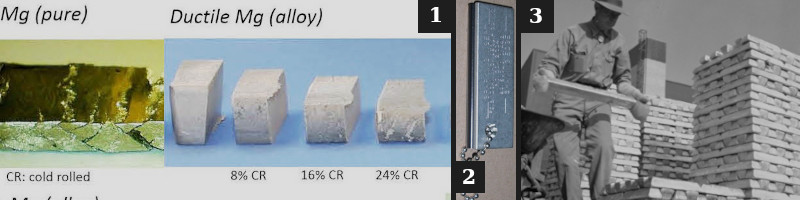
1. Alloys compared to elemental Mg.
2. Magnesium is used in Fire Starter kits [71].
3. Magnesium metal is different from the Magnesium ions in the soil.
Wikimedia Commons [68a].
Magnesium is found within all living organisms due to its electrical charge and size, which control the behavior of the major energy transporter molecules: ATP and ADP. It is very important to the function of cells [72]. Magnesium inside cells is found as a cation, Mg2+ . It bonds with other anions ( – ), negatively charged atoms. These conditions occur when both atoms have more or less electrons than protons.
Calcium

Wikimedia Commons [69a].

Wikimedia Commons [70a].
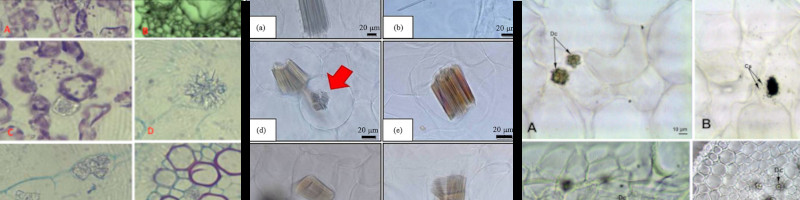
Left: Ruby dock (Rumex vesicarius) leaves and stems.
Middle: Porang (Amorphophallus muelleri) corm.
Right: Hops (Humulus lupulus) leaves and stems.
El-Zaidy 2021, Chairiyah et al 2016, and Konyar et al 2014 [73][74]
[71a].
Dr. Jiwan Palta said it succinctly, “Calcium is nature’s strength.” Calcium is in shells of land and sea creatures, and in the bones of animals.
Calcium oxylate crystals form within the tissues of some plants like the Swiss Cheese plant (Monstera deliciosa) can damage the inside of your mouth and gut. Calcium carbonate is another chemical found in many kinds of limestone bedrocks, and also in chalk.
Calcium is also important in metabolism of all living organisms [75][76][77]. The charge of Ca2+ ions and its size, in comparison to Mg2+ or Zn2+ or Cd2+ ions, allow it to interact with oxygen donating molecule groups like alcohols (see here) and others [78]. For this reason, Calcium plays a role in the biosynthesis of lignin, among many other organic molecules. Lignin is the molecule around the cell wall, which gives wood its strength. Studies of Poplar trees (Populus sp.) and their response to calcium-availability show it can affect the strength and quality of wood [79].
Sulfur

Adapted from Welcome1To1The1Jungle’s upload.
Wikimedia Commons [72a].

Middle: Sulfur-containing compounds produce bad smells, often associated with the smell of rotten eggs.
Right: Sulfur harvests, Mount Ijen, East Java, Indonesia.
Wikimedia Commons [73a].
Sulfur (S) plays a role in the metabolism of plants [80]. Many of the qualities we associate with onions (Allium cepa) and other Alliums with strong odors are because of Sulfur containing secondary metabolites.
When you cut an onion, a sulfur containing compound is evaporated into the air and reacts with the water in your eyes to produce sulfuric acids at low concentrations, which cause them to irritate and make us cry. Workers in kitchens that cut onions frequently are advised to wear protective eye gear to prevent eye damage. Plant breeders are interested in breeding onions with less of these compounds to protect workers. Irwin Goldman is one breeder who researched the medicinal role of these compounds and their positive effect on heart health.
In the past, flowers of sulfur was a powdered Sulfur and common pesticide used to fight fungal diseases. This dust was applied to plants. Sulfur smells like rotten eggs. Think of how smelly that would’ve been!
Micronutrients

1. Micronutrients are sold as plant nutritional supplements. They can also be called trace minerals or trace elements.
2. Peat and sphagnum moss (Spagnum spp.) have traditionally been harvested and used for various purposes. In horticulture, they form the bulk constituent of soil-less growing media used in potting soils. These can lack nutrients.
Peat harvest in Orkney Islands, Scotland, UK.
3. Hydroponics is a soil-less cultivation technique, which directly adds water to roots and can also require added fertilizers. Aquaponics utilizes fish to produce nutrients in water.
Urbanexotic’s greenhouse, Ahmedabad, India.
4. Cut-flowers sometimes have inputs (ex: citric acid, sugar) in their water to preserve flower quality in transit or at destination.
California, USA, 1999.
5. Spectrometry allows horticulturalists, botanists, pedologists, and other scientists to analyze the presence of atomic elements within a sample.
Cedara College of Agriculture, Hilton, South Africa.
6. Early scientists like Franklin Hiram King (FH King) studied agricultural practices in China, Korea, and Japan to understand how these societies managed to cultivate and not deplete their soil fertility for the last 4,000 years.
Piles of compost, Shantung, China, 1900.
Wikimedia Commons [74a].
Iron

Rezvan Motahari,
Wikimedia Commons [75a].

Caused by nutrient deficiencies, diseases or damage. Plants in soils with a lot Ca (calcareous soils), introduced crops in soils without as much Iron, or plants in containers can exhibit chlorotic leaves due to Iron-deficiency.
Wikimedia Commons [76a].
Iron (Fe) has been smelted from ore and made into metal tools by humans around the world [81]. Metal iron forms rust when Iron oxidizes. Iron oxides, like Fe(OH)3, have a red color. Soils with iron will oxidize more around the tropics, where soils experience more weathering through heat and rain.
In mammals, but not insects, it constitutes a part of oxygen-carrying organic molecules like hemoglobin in blood. For plants, Iron plays a role in the biosynthesis of chlorophyll, a green pigment molecule used in photosynthesis.
When prepared in fertilizers, Iron is prepared into a powder with other complex molecules which prevent oxidation from occurring before the Iron reaches the plant. These molecules are known as chelators (kee-late-ors), and the mixed product as chelated iron or iron chelate.
Copper

Wikimedia Commons [77a].
Copper (Cu) is used in electrical wires and the electronic devices we use to plan our gardens, communicate, and learn. Copper, although it is a micronutrient, plays a significant role in horticulture, because of its chemical properties [82][83].
Copper is toxic to microorganisms. Plants have some ways to mitigate copper toxicity. Because copper is lethally toxic to microbes, it is used in fungicides. It is also present in the ionic molecule of certain nitrate fertilizers, giving it a blue color.
Sodium

Wikimedia Commons [78a].

1. Plant communities along the coast of Langeux, Brittany, France.
2. Salt marsh near Huntington Beach, California, USA.
3. Sea Purslane (Halimone portulacoides), Marismas del Río Palmones, Andalucia, Spain [84].
4. Sea aster (Tripolium pannonicum) near Saint-Brieuc, Brittany, France.
5. Sea plantain (Plantago maritima) near Saint-Jean-de-Luz, Pyrénées-Atlantiques, France.
Wikimedia Commons [79a].
Many people might be surprised to learn that Sodium (Na) is a micronutrient. We do not recommend adding any salt to your growing media, because the requisites for Sodium in plants are so small they are almost always fulfilled. What people should be concerned about is the negative consequences of having too much Sodium.
Sodium is a very reactive substance and therefore is most often bonded to another element. These bonded crystalline molecules are called salts. Other elements form salts for similar reasons, yet we are most familiar with table salt: Sodium chloride (NaCl). The kind of Sodium chloride we are concerned about has been traditionally mined from quarries, which were deposited by ancient oceans. Other methods of harvesting include evaporating salty water from the ocean or other sources. Some cultures without access to salt mines and sea water have burned certain plants rich in salts and consume their ashes, since humans require salt in their diets. Evaporative methods are almost always used to produce salt for culinary purposes. Mining methods are used for industrial production used in roads and sidewalks in high quantities, which has contaminated lakes and damages sensitive plants.

1. Hitchable salt spreaders in Moscow, Russia.
2. Example of a rock salt dispersal mechanism.
3. Road salt machines deployed in snow emergency in Madrid, Spain.
4. Manual salt spreaders in New York City, NY, USA.
5. Salt brine applicators are being researched and deployed in Wisconsin in order to lessen salt spread []. In this case, it is being used preemptively. Virginia, USA.
Wikimedia Commons. Flickr [80a].
In colder climates with snowy icy winters, salt is applied to roads to turn ice into slush by changing the water’s freezing point. This salt can damage lawns, roadside flora, and sensitive ecosystems downstream of the melting snow. Because plants rely on osmosis to intake water, salt contamination can reverse this passive mechanism and cause plants to die of dehydration. Chloride ions in salt can also have damaging effects to the biology of plants.
For similar reasons, salts of fertilizers can also have the same effect on plants. Farmers call this osmotic damage fertilizer burn. Like table salt, these salts can dissolve in water and drain downstream into creeks, rivers, and lakes, causing problems of their own, such as overgrowth of aquatic plants.
Regulation
Governments regulate what inputs can be used in agriculture to protect people and the environment. Governments also regulate how those substances are used and where they come from. Research-informed categories are used to try to understand the effects of these substances on humans (ex: pregnant women, babies, children, adults), animals (ex: pets, farm animals, wild animals, insects), plants (ex: crops, wild populations) and the environment (ex: forests, wetlands, lakes, rivers, air). Regulations manage thousands of inputs.

Left to right: Argentina, Australia, Germany, Brazil, Canada, East Africa (Kenya), USA, Ukraine, Israel.
Wikimedia Commons [81a].
The words, Bio or organic or natural, can be used to describe how to grow in a minimalistic life-friendly way, to grow without the use of chemicals (artificial chemicals), to grow traditionally, or to grow naturally [85].
Because there are now many substances used in modern agriculture, some natural with very negative consequences (ex: rotanone, poisons fish; nicotine, poisons insects) and some artificial with many benefits (ex: chlorine or alcohol, for sanitation), regulations try to create a system to define these big ideas into organized enforceable realities. Since some of these substances can be mined, and not synthetically produced, some of them are allowed in organic food production systems by regulatory organizations [86][87].
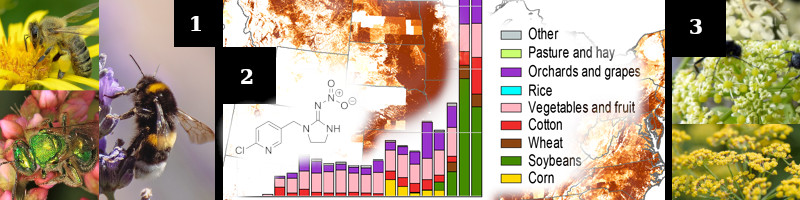
1. “Save the bees”, people think about honey bees (Apis mellifera), yet there are sweat bees (Halictidae family) and bumblebees (Bombus spp.). These are recognizable and easier to use as examples, but are not the only insects at risk. Insect diversity is great.
2. A molecule of imidacloprin (common neonicotinoid), bar chart of total imidacloprin use per year and crop (1994-2011, max value: ~1.5×10³lbs, top crop: soybeans), and map of use in the USA (max value: >0.24lbs/mi²).
3. Scientists are promoting IPM (Integrated Pest Management) as an industry approved methodology, which encourages critical analysis, use of cultural controls or non-chemical controls, and the management of chemical applications.
One possible alternative to chemical pesticides is planting native plants to certain families (ex: Apiaceae), which attract and sustain populations of beneficial insects (beneficials). Beneficials can consume, parasitize, or compete with pests.
Another possible alternative is to plant trap crops, which are planted to attract pests, away from the desired crop.
Watch a video about it here.
Wikimedia Commons [82a].
In the USA, the USDA regulates the term ‘Organic’ or similar terms used in any product label, such as food, fertilizers, and other garden amendments. Before someone can use this word in a product or receive a certified USDA Organic label, they must farm for three years with permissible inputs as defined by the USDA and be randomly inspected for compliance. Non-organic food is said to be ‘conventionally grown’. Conventional systems are also regulated by the USDA and FDA but allow a broader range of accepted substances.
Because the list of permissible organic inputs is long, has some exceptions, requires time and money, farmers who grow without inputs or with traditional inputs may coordinate with organizations called CSA’s (Community-supported Agriculture) or other methods of business, but still cannot use the term ‘organic’. Regulatory agencies inspect and try to control this.
Other countries and governmental bodies, like the European Union, have different regulations, and can decide which countries they will accept certain goods from with treaties and agreements. Different countries can also control or safeguard their inhabitants by using different patent systems, and labeling laws.
Some people argue against regulations because it tries to control behavior, its confusing, they see corruption, and the false power it can give business competitors. Organic food can still be affected by food-borne diseases, can still contribute to dietary issues, and can be overly contentious issue that causes people to mistreat each other [88]. People seem to want good on both sides of the argument, yet organic agriculture has been favored by many for its emphasis on applying practices with less negative consequences.
Contaminants
Not all elements are conducive to life [89][90]. Some are naturally there in the soil, and for that reason, some places on Earth are barren or have environments where organisms have to adapt or die. Those that survive by working together or inherit new traits have offspring and their offspring evolve at each generation.
Other sources of detrimental elements come from human activity. Since ancient times, humans have explored how to control materials and create new substances. After the industrial revolution, the productivity improved, yet also did the potential for harm. Today, industries, municipalities, businesses and consumers need to manage their use of chemicals or suffer negative consequences.
Sometimes the scope of protection is limited to the factors involved in the success of their operation and not the needs of people or the environment. For the last 100 years, the world has seen a dramatic rise in pollution, which threatens the Web of Life, which we and our economy are a part of.
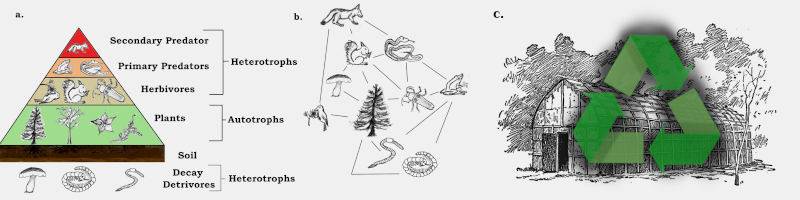
a. Trophic Pyramid – Classifies the eating habits (-trophy) of organisms unidirectionally.
Heterotrophs (eats many things) are composed of herbivores (plant-eaters), primary predators (herbivore eaters), and secondary predators (predator eaters). The highest tier of secondary predators are classified as keystone species or apex predators. On the other end of the spectrum are detrivores (eats to decompose), which live in the soil, the primary substratum for the autotrophs (eats on its own), many of which use sunlight as the necessary metabolic catalyst.
b. Trophic Web – Classifies eating habits multidirectionally.
Since diets and relationships between organisms are complicated, these type of diagrams can often better portray the web of interactions between many species or other taxa.
c. Ecology and Economy – How nature and money are related.
Shown in picture: Recycle symbol imposed over an Iriquoian longhouse.
Both the word ecology (study of relationships in nature) and economy (system of exchange, usually goods and services) come the Greek word οἶκος (oikos, house). Like a house, especially a longhouse where many people live together, everything in the world is connected and everything it in it studied through different measurements.
Wikimedia Commons [83a].
To avoid polluting the environment, humans need to identify where the contaminants are coming from. There two categories: point-source pollution and nonpoint-source solution. Point-source pollution occurs when there is an easily identifiable source, like a factory or broken sewage pipe. Nonpoint-source pollution occurs when there are many causes, like the draining of pollutants into drains after it rains.
Lead (Pb)

2. Galena (PbS) – Mineral with high quantity of Lead sought after during lead mining. It can be found abundant in certain geological formations, hence why it is the State Mineral of Kansas, Missouri, and Wisconsin, and the name of the City of Galena, Illinois.
3. Lead-acid batteries – Large battery made from plates of Lead and used as car batteries. Important to properly dispose of any lead-acid battery, even though it is covered by plastic, since it can decompose, leak, and contaminate.
4. Lead bullets – Because of Lead’s malleability and abundance, it has been used as metal for bullets, although it is less common nowadays. This is a reminder of how warfare is not only detrimental to human life, but also leaves a lasting negative impact on the environment.
5. Fishing weights – For the same reason, fisherman weights used to hold down the bait below a floating bobber are a source of contamination. Today, people are asked to donate their old fisherman weights to appropriate locations for proper disposal.
6. Lead oxide (PbO) – Lead oxides have different color than the typical grey color. Hence, why it is necessary for regulatory bodies to track what products have Pb in them.
7. Lead paint from a house built in 1948 – Because of the color of Lead oxides, Lead paint was popular choice for painting buildings white or light colors before the 1970’s. For this reason, and lead piping, many urban areas have significant lead-exposure issues that affect their inhabitants and contaminate soils around the foundation of buildings, where gardens are placed. Contaminated food is one way Lead-exposure can occur.
Wikimedia Commons [84a].
Lead (Pb) has another curious abbreviation, which comes from the Latin word Plumbum, and was also called Plumbum nigrum, since the Romans couldn’t distinguish Tin (Sn) from Pb, except for its color.
Chronic lead exposure causes neurodegenerative disorders, damage to vital organs, and correlates with gun violence statistics. Therefore, it has become one of the most studied contaminants [91][92][93][94]. It can naturally exist in certain geological formations and spread through mining. It can also exist within cities, since old paints and pipes often used Pb.
Lead testing of soils is one of the main tests offered by university extension labs. In certain cities where Lead exposure is a greater risk, like Milwaukee, Wisconsin, parents are encouraged to test their kids for blood-levels of lead. Some anecdotes say unapproved soil testing in places like Milwaukee can receive criminal charges, presumably because cities wish to obfuscate the severity of their lead pollution, from natural or human sources.
Home gardens near the foundation of homes are one of the most common sources of Pb contamination. Pb enters the tissues of plants consumed as food. For this reason, some people advise test for Pb when planning a garden.
Lead was discovered in the White House gardens and its soil had to be properly discarded and replaced [95]. Such procedures are expensive and might not be available to home owners of houses built before 1970’s. In the USA, the government wants to subsidize and fund the costs of replacing pipes that deliver drinking water [96].
Remediation
Scientists and land stewards wish to understand how they can remove the contaminants from an environment. The best method to avoid this problem is to stop them at their source. Many times, further we are from the source, the harder it is to fix the problem, because of entropy, the universal law which describes how things change over time.
The second best method is to remediate the environment with a solution that causes the least amount of negative consequences. Sometimes scientists use chemicals in order to chemically solve another chemical pollutant issue. These chemicals can have their own consequences and contribute to other problems.

polychlorinated dibenzo-p-dioxins (PCDDs)
polychlorinated dibenzofurans (PCDFs)
and some polychlorinated biphenyls (PCBs)
A. Graph shows decrease in airborne PCDDs and PCDFs from 1990s until 2015, across observation sites in United Kingdom, South Korea, Spain, Taiwan, and Japan.
B. Chemical structure of PCDDs – PCDDs and PCDFs are anthropogenic contaminants, which can cause cancer and other illnesses.
C. Danang Airport Remediation structure – US and Vietnam governments worked together to remediate contaminated soils from Vietnam War. Soils were surveyed, contaminated soils were then placed inside a structure and treated with heat to destroy the contaminants.
Learn more about it here. [97][98][99][100][101]
Wikimedia Commons [85a].
While planting a plant to “soak up” the contaminants might seem like a good idea, it can also have negative consequences if the contaminant is now exposed to pollinators or is not properly disposed. By studying the physiology and ecology of plants, we can better ascertain what role plants have in remediation.
Some issues aren’t caused by dangerous chemicals, but by changes in naturally occurring organic substances in soils because of erosion, drought and disturbances. These can simply resolved if one studies plants, particularly native plants, so to not introduce new consequences, and their characteristics (morphology, physiology, ecology). Once studied, landscapes can be engineered through the successive planting of new species (1. one type species first, 2. another type later once the first type has influenced the soil, etc…).
Conclusion
The organization of the elements in the world is complicated and requires dedicated study. Many have attempted to understand it all. If we were to talk about the origin of an element like Iron, one we have coursing through our bodies, we’d need to go back to the life cycle of stars.
In plant science, a holistic approach could help us focus on the relationship between each element rather than divide it all into a million parts and pieces. Such a holistic approach would help us see plants, not as lego-like structures we can mess with, but a symphony of harmonies. If we can understand how plants function, we can use this information to describe how sustainable cultivation methods work and why describe why some innovations cause harm.

Sounds carry information, like a signal, which rely on structure to communicate information, just like how plants use the elements.
Music as a Metaphor
Just like music, nature works together and functions because it accomplishes special juxtaposed patterns.
The Elements
The atoms, the elements, the chemicals, the organisms, they all contribute to a global function.
Wikimedia Commons [86a].
Philosophical approaches to this body of knowledge are just as important as the knowledge itself. How can we know what to do, and when not do it [102]? When is an input helpful, and when is it not? Of course, the answers of to these questions are in the never ending quest of evaluating the evidence of scientific research.
Most importantly, PlantResearchOrg emphasizes cooperation and the Golden Rule. Interpersonal and self-help skills like empathy, sympathy and compassion are necessary to manage these complicated and sensitive topics. What’s done is done. Now we decide the quality of our action forward .
Author: Gustavo Meneses
Published: 2025-01-08
Revised: 2025-01-08
Read more
[I] Clayton, Michael W. “Cross section of a leaf of Nerium oleander showing stomatal crypt”. UWDC. Accessed Dec 7, 2023. https://digital.library.wisc.edu/1711.dl/SJLPA2KTSOOC48T
[II] “C3 C4 images.htm”. Jun 19, 2013. Biology 111 Lab. Yvonne Vaillancourt Biology Department. UMass Boston. Accessed Dec 7, 2023. http://www.faculty.umb.edu/yvonne_vaillancourt/Biology/
[III] Lakna. “What is the Difference Between Microbes and Microorganisms”. Apr 30, 2019. Pediaa. Accessed Dec 16, 2023. https://pediaa.com/what-is-the-difference-between-microbes-and-microorganisms/
[IV] Peganum. “Seed sowing, resowing, and pricking out”. Album. Flickr. Accessed July 2, 2024. https://www.flickr.com/photos/peganum/albums/72157663392571465/
[V] Krutul, D. et al. “METALS ACCUMULATION IN SCOTS PINE (PINUS SYLVESTRIS L.) WOOD AND BARK AFFECTED WITH ENVIRONMENTAL POLLUTION”. Jan 2017. Wood Research, vol 62, no. 3, pp.353-364. Department of Wood Science and Wood Protection. Warsaw University of Life Sciences. ResearchGate. https://www.researchgate.net/publication/322027112_METALS_ACCUMULATION_IN_SCOTS_PINE_PINUS_SYLVESTRIS_L_WOOD_AND_BARK_AFFECTED_WITH_ENVIRONMENTAL_POLLUTION
[VI] “First direct imaging of small noble gas clusters at room temperature”. Jan 11, 2024. Eureka Alert. Nature Materials. Accessed Feb 03, 2024. http://dx.doi.org/10.1038/s41563-023-01780-1
- Thanks to Project Civil Cultural’s news aggregator. Website: https://civilcultural.wordpress.com/ Discord: https://discord.gg/xNeTXB8S
See more
[Ia] Chorover, J. Kretzschmar, R. Garcia-Pichel, F. Sparks, DL. “File:Earth Magnetic Field Declination from 1590 to 1990.gif”. Oct 1, 2007. Wikimedia Commons. Accessed Feb 02, 2024. https://commons.wikimedia.org/wiki/File:Earth_Magnetic_Field_Declination_from_1590_to_1990.gif
[IIa] Tdadamemd. “File:Solar System scaled to a football field.png”. May 7, 2012. Wikimedia Commons. Accessed Dec 1, 2023. https://commons.wikimedia.org/wiki/File:Solar_System_scaled_to_a_football_field.png
[IIIa] OptimusPrimeBot. “File:Three Steps to the Hubble Constant (2019-25-4489).tif”. Apr 25, 2019. Wikimedia Commons. Accessed Dec 1, 2023. https://commons.wikimedia.org/wiki/File:Three_Steps_to_the_Hubble_Constant_(2019-25-4489).tif
[IVa] “File:Terrestial Planets internal en.jpg”. Aug 13, 2013. NASA. Wikimedia Commons. Accessed Feb 02, 2024. https://commons.wikimedia.org/wiki/File:Terrestial_Planets_internal_en.jpg
[Va] “File:Czone chorover et al catalina jemez czo.png”. Oct 1, 2007. Wikimedia Commons. Accessed Feb 02, 2024. https://commons.wikimedia.org/wiki/File:Czone_chorover_et_al_catalina_jemez_czo.png
[VIa] Kelvinsong. “File:C4 photosynthesis is less complicated.svg”. May 29, 2013. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:C4_photosynthesis_is_less_complicated.svg
[VIIa] Ninghui Shi. “File:Cross section of Arabidopsis thaliana, a C3 plant..jpg”. Sept 13, 2013. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:Cross_section_of_Arabidopsis_thaliana,_a_C3_plant..jpg
References
[1] Stathern, Paul. “Mendeleyev’s Dream: The Quest for the Elements”. pp.127-138. Apr 30, 2001. Gardners Books. 978-0140284140. https://www.librarything.com/work/408287
[2] “Timeline of NRAO History”. National Radio Astronomy Observatory (NRAO). Accessed Jan 8, 2024. https://www.nrao.edu/archives/nrao-timeline
[3] “The History of Radio Astronomy”. National Radio Astronomy Observatory (NRAO). Accessed Jan 8, 2024. https://public.nrao.edu/radio-astronomy/the-history-of-radio-astronomy/
[4] “The Science of Radio Astronomy”. National Radio Astronomy Observatory (NRAO). Accessed Jan 8, 2024. https://public.nrao.edu/radio-astronomy/the-science-of-radio-astronomy/
[5] “What is the meaning of יוֹם (yowm) in Bereshit?”. Judaism. StackExchange. Website. Accessed Sep 29, 2021. https://judaism.stackexchange.com/questions/10079/what-is-the-meaning-of-%D7%99%D7%95%D6%B9%D7%9D-yowm-in-bereshit
- Religious topics are not entirely refutable, therefore should not be interpreted the same as scientific evidence. This resource is helpful to gain insight into the educated opinions of others about non-scientific matters relevant to the concepts of the origin of the universe.
[6] Jump, Tyler. “Measuring the Age of the Universe”. Oct 17, 2018. News. Center for Astrophysics. Smithsonian Museum & Harvard College. Accessed Jan 8, 2024. https://www.cfa.harvard.edu/news/measuring-age-universe
[7] Cosmology Space. “Brian Cox – Can we see the ACTUAL Big Bang”. Jan 22, 2024. YouTube Shorts. Accessed Jul 8, 2024. https://youtube.com/shorts/B1QKhAUaUr8?feature=shared
[8] “What are the different types, or classes, of flares?”. Space Weather Monitors. SOLAR Center. Stanford. Accessed Dec 1, 2023. https://solar-center.stanford.edu/SID/activities/flare.html
[9] “Details and graphs for CME0150”. Cactus. Accessed Dec 1, 2023. https://www.sidc.be/cactus/catalog/LASCO/2_5_0/2012/08/CME0150/CME.html
[10] “CMEs deteced by Cactus”. Cactus 2.5.0. Accessed Dec 1, 2023. https://www.sidc.be/cactus/catalog/LASCO/2_5_0/2012/08/latestCMEs.html
[11] “Nucleosynthesis – How Elements Are Made”. Science Notes. Accessed Nov 23, 2023. https://sciencenotes.org/nucleosynthesis-how-elements-are-made/
[14] St. John, James. “BYER SANDSTONE”. JSJ Geology. Accessed Mar 5, 2024. http://jsj-geology.net/Logan-Formation.htm
[15] “ḥwt-kꜣ-ptḥ”. Wiktionary. Accessed Feb 15, 2024. https://en.wiktionary.org/wiki/%E1%B8%A5wt-k%EA%9C%A3-pt%E1%B8%A5
[16] Weinstein, Joseph. “We Were Slaves to the Hyksos”. 2021. The Torah. Accessed Feb 15, 2024. https://www.thetorah.com/article/we-were-slaves-to-the-hyksos-in-egypt
[17] “2345. chum”. Strong’s Concordance. Bible Hub. Accessed Feb 15, 2024. https://biblehub.com/hebrew/2345.htm
[18] “Genesis 30”. The Contemporary Torah. Sefaria. Accessed Feb 15, 2024. https://www.sefaria.org/Genesis.30?lang=bi&aliyot=0
[19] Pergament, Wesley. “BOOM! The Story Behind Gunpowder”. Sept 10, 2017. The Daily China. Accessed Feb 15, 2024. https://thedailychina.org/boom-the-story-behind-gunpowder/
[20] Theobald, Ulrich. “Zhouyi cantong qi”. Aug 17, 2010. ChinaKnowledge. Wikimedia Commons. Accessed Feb 15, 2024. http://chinaknowledge.de/Literature/Daoists/zhouyicantongqi.html
[21] Wei Boyang. Predagio, Fabrizio. “Zhouyi cantong qi”. The Golden Elixir. PDF. Accessed Feb 15, 2024. https://www.goldenelixir.com/files/Zhouyi_cantong_qi_Text.pdf
[22] Guinta, Carmen. “Classic Chemistry”. Le Moyne College. Accessed Feb 28, 2024. https://web.lemoyne.edu/giunta/
[23] Rogers, E. Stovall, I. Jones, L. Chabay, R. Kean, E. Smith, S. “Fundamentals of Chemistry”. 2000. University of Illinois Urbana-Champaign. Accessed Feb 28, 2024. http://www.chem.uiuc.edu/webFunChem/GenChemTutorials.htm
[24] Nutt, David. “Cornell researchers see atoms at record resolution”. May 20, 2021. Cornell University. Accessed Dec 2, 2023. https://news.cornell.edu/stories/2021/05/cornell-researchers-see-atoms-record-resolution
[25] Gesing, T. Uecker, R. Buhl, JC. “Refinement of the crystal structure of praseodymium orthoscandate, PrScO3”. Apr 2009. Zeitschrift für Kristallographie. New Crystal Structures, Issue 224. pp.365-366. ResearchGate. http://dx.doi.org/10.1524/ncrs.2009.224.14.38
[26] Helmenstine, Anne. “What Is a Chemical? Definition and Examples”. Oct 15, 2023. ScienceNotes. Accessed Feb 15, 2024. https://sciencenotes.org/what-is-a-chemical-definition-and-examples/
[27] “Who is Mikhail Tsvet?”. Oct 29, 2014. Chromatography Today. Accessed Dec 15, 2023. https://www.chromatographytoday.com/news/gc-mdgc/32/breaking-news/who-is-mikhail-tsvetnbsp/32304
[28] Helmenstine, Anne. “Adsorption vs Absorption – Differences and Examples”. Jul 15, 2021. Science Notes. Accessed Dec 15, 2023. https://sciencenotes.org/adsorption-vs-absorption-differences-and-examples/
[29] Buie, John. “Evolution of Chromatography Columns”. May 3, 2011. Product News. LabManager. Accessed Dec 15, 2023. https://www.labmanager.com/evolution-of-chromatography-columns-18674
[30] Trapp, Dave.”The Chemical Elements”. Dec 31, 2009. Sequim Science. Accessed Feb 28, 2024. http://d1068036.site.myhosting.com/Elements/elements.html
[31] “elements2.pdf”. CHM448. Marshall University. Accessed Dec 2, 2023. http://www.science.marshall.edu/castella/chm448/elements2.pdf
[32] “Properties”. Ptable. Accessed Dec 19, 2023. https://ptable.com/#Properties
[33] Helmenstine, Anne Marie. “Why Is the Carbon Cycle Important?”. Oct 24, 2022. ThoughtCo. Accessed Dec 2, 2023. https://www.thoughtco.com/carbon-cycle-important-607597
[34] Miao, L. Chmielewski, A. Mukherjee, D. Alem, N. “Picometer-Precision Atomic Position Tracking through Electron Microscopy”. Jul 2021. J Vis Exp. Vol 3, no. 173. https://pubmed.ncbi.nlm.nih.gov/34279511/
[35] Bio_Lyric404. “Sigma and Pi Bonds”. Sep 1, 2022. CASEY-HAS-ALLEN. Accessed Dec 5, 2023. https://casey-has-allen.blogspot.com/2022/08/sigma-and-pi-bonds.html
[36] “Why Is Carbon So Important In Biology? Key Element Of Life On Earth”. Jan 1, 2020. Biology Junction. Accessed Dec 2, 2023. https://biologyjunction.com/why-is-carbon-so-important-in-biology/
[37] Campbell, Leah. “Explained: The sugar coating of life”. Dec 1, 2023. MIT News. Massachusetts Institute of Technology (MIT). Accessed Dec 2, 2023. https://news.mit.edu/2023/explained-glycoscience-carbohydrates-1201
[38] Wang, C. Guo, L. Yixue, L. Wang, Z. “Systematic Comparison of C3 and C4 Plants Based on Metabolic Network Analysis”. Dec 12, 2012. BMC Systems Biology. Springer Link. Vol 6, no. S9. https://link.springer.com/article/10.1186/1752-0509-6-S2-S9
[39] Khoshravesh, R. Stata, M. Adachi, S. Sage, TL. Sage, RF. “Evolutionary Convergence of C4 Photosynthesis: A Case Study in the Nyctaginaceae”. Nov 2, 2020. Front. Plant Sci. Sec. Plant Systematics and Evolution. Vol 11. https://doi.org/10.3389/fpls.2020.578739
[40] Herrero, Joaquín. “Plantas CAM”. Flora de Iberia. Accessed Dec 5, 2023. https://floradeiberia.com/28/plantas-cam/
[41] Ritchie, Raymond J. “Photosynthesis in the Blue Water Lily (Nymphaea caerulea Saligny) using PAM fluorometry”. Feb 2012. Int. Journal of Plant Sci., vol 173, no. 2, pp.124-136. http://dx.doi.org/10.1086/663168
[42] dana1981. “Plants cannot live on CO2 alone”. Skeptical Science. Accessed Dec 5, 2023. https://skepticalscience.com/print.php?r=284
[44] “water strider”. BugGuide Advanced Search. BugGuide. Accessed Dec 15, 2023. https://bugguide.net/adv_search/bgsearch.php?user=&taxon=&description=water+strider&location%5B%5D=WI&county=&city_location=&adult=&immature=&male=&female=&representative=
[43] “Adhesion and Cohesion of Water”. Jun 5, 2018. Water Science School. United States Geological Survey (USGS). Accessed Dec 15, 2023. https://www.usgs.gov/special-topics/water-science-school/science/adhesion-and-cohesion-water
[45] Barak, Phillip. “Essential Elements for Plant Growth”. Jan 11, 1999. Dept. of Soil Science. University of Wisconsin-Madison (UW-Madison). Accessed Dec 19, 2023. https://soilsfacstaff.cals.wisc.edu/facstaff/barak/soilscience326/essentl.htm
[46] Mentzer, Robert. “The oldest known tree in Wisconsin is a 1,300-year-old cedar growing from a cliff”. Wasau Daily Herald. Accessed Feb 28, 2024. https://eu.wausaudailyherald.com/story/news/2019/04/22/oldest-tree-wisconsin-1300-year-old-red-cedar-niagara-escarpment-brown-county-greenleaf-doug-larson/3428841002/
[47] Uchida, R. “Chapter 3: Essential Nutrients for Plant Growth: Nutrient Functions and Deficiency Symptoms.” 2000. Silva, JA. Uchida, R. “Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture”. College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa. Accessed Mar 12, 2024. https://www.ctahr.hawaii.edu/oc/freepubs/pdf/pnm3.pdf
- Hawaii’s recently formed soils are a good example for outdoor environments where introduced crops can experience nutrient deficiencies.
[48] Knuteson, James A.”THE VARIATION OF SPODIC CHARACTERISTICS IN NORTH CENTRAL WISCONSIN”. May 1979. University of Wisconsin – Stevens Point. Accessed by Feb 28, 2024. https://minds.wisconsin.edu/bitstream/handle/1793/79525/Knuteson.pdf?sequence=1
[49] “Keys to Soil Taxonomy”. Natural Resources Conservation Service (NCRS). United States Department of Agriculture (USDA). Accessed Feb 28, 2024. https://www.nrcs.usda.gov/resources/guides-and-instructions/keys-to-soil-taxonomy
[50] Hewitt, Allan. “Story: Soils”. TE ARA – the Encyclopedia of New Zealand. Accessed Feb 21, 2024. https://teara.govt.nz/en/photograph/12281/soil-texture
[51] “Understanding Soil”. Landscape for Life. Harnett County Center. Extension. North Carolina (NC) State University. Accessed Feb 21, 2024. https://harnett.ces.ncsu.edu/wp-content/uploads/2020/09/Soils.pdf?fwd=no
[52] “The Twelve Orders of Soil Taxonomy”. Natural Resources Conservation Service (NRCS). USDA. Accessed Dec 19, 2023. https://www.nrcs.usda.gov/resources/education-and-teaching-materials/the-twelve-orders-of-soil-taxonomy
[53] Arancibia, Ramón A. “Soil Steaming to Reduce the Incidence of Soil-borne Diseases, Weeds and Insect Pests”. Nov 24, 2020. Missouri Produce Growers (MPG). Integrated Pest Management (IPM). University of Missouri. Accessed Mar 15, 2024. https://ipm.missouri.edu/MPG/2020/11/steaming-RA/
[54] “The Atmosphere”. National Oceanic Atmospheric Administration (NOAA). Accessed Dec 19, 2023. https://www.noaa.gov/jetstream/atmosphere
[55] Bornet, Rémi. “What is clay in geology and mineralogy?” Le Comptoir Géologique. Accessed Feb 1, 2024. https://www.le-comptoir-geologique.com/clay-glossary.html
[56] Franzen, DW. Bu, H. “North Dakota Clay Mineraology Impacts Crop Potassium Nutrition and Tillage Systems”. Sep, 2023. SF1881. North Dakota State University (NDSU). Accessed Feb 01, 2024. https://www.ndsu.edu/agriculture/ag-hub/publications/north-dakota-clay-mineralogy-impacts-crop-potassium-nutrition-and-tillage
-See ‘Potassium availability in soils’
[57] “Chernozem”. Soil Classification: Soil Orders of Canada. Accessed Dec 19, 2023. https://classification.soilweb.ca/chernozem/
[58] Silva, EM. “Source: UNIV OF WISCONSIN submitted to”. Accession no. 1011812. Project no. WIS01973. United States Department of Agriculture (USDA). Research, Education & Economics Information System (REEIS). Accessed Dec 19, 2023. https://portal.nifa.usda.gov/web/crisprojectpages/1011812-unraveling-the-soil-microbiome-integrating-crop-production-soil-management-and-metagenomics-to-bring-science-to-practice.html
[59] “Lankau Lab.”. UW Plant Pathology. UW-Madison. Dec 19, 2023. https://lankau.russell.wisc.edu/
[60] Castro, Mercedes. “Ciclo del fósforo”. Jul 21, 2021. Lifeder. Accessed Feb 01, 2024. https://www.lifeder.com/ciclo-fosforo/
[61] Bralower, Tim. “The Phosphorus Cycle and Human Management of Soils”. Food and the Future Environment. Pennsylvania State University. Accessed Mar 5, 2024. https://www.e-education.psu.edu/geog3/node/1176
[62] Hyland et al. “Phosphorus Basics – The Phosphorus Cycle”. 2005. Agronomy Fact Sheet Series, Fact Sheet 12. Cornell University Cooperative Extension. Nutrient Management Spear Program. Department of Crop and Soil Sciences. College of Agriculture and Life Sciences. Cornell University. PDF. Accessed June 28, 2024. http://nmsp.cals.cornell.edu/publications/factsheets/factsheet12.pdf
[63] Khan, F. Siddique, AB. Shabala, S. Zhou, M. Zhao, C. “Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses”. 2023. Plants (Basel), vol 12, issue 15, 2861, Aug 2023. https://doi.org/10.3390%2Fplants12152861
[64] Castro, Mercedes. “Ciclo de potasio: concepto, etapas e importancia”. Nov 11, 2020. Lifeder. Accessed Feb 01, 2024. https://www.lifeder.com/ciclo-del-potasio/
[65] Hennings, Jull. Lynch, Harry. “Powerful Pot Ash”. July 27, 2022. EarthDate. Accessed Jun 28, 2024. https://www.earthdate.org/episodes/powerful-pot-ash
[66] Somarin, Ali. “Potash: A Look at the World’s Most Popular Fertilizer”. June 26, 2014. ThermoFisher Scientific. Accessed June 28, 2024. https://www.thermofisher.com/blog/mining/potash-a-look-at-the-worlds-most-popular-fertilizer/
[67] Fromm, Jörg. “Wood formation of trees in relation to potassium and calcium nutrition”. May 2, 2010. Tree Physiology, vol. 30, issue 9, Sep 2010, pp. 1140-1147. https://doi.org/10.1093/treephys/tpq024
[68] Johnson et al. “Potassium in plants: Growth regulation, signaling, and environmental stress tolerance”. Plant Physiology and Biochemistry, vol 172, Feb 1 2022, pp. 56-69. https://doi.org/10.1016/j.plaphy.2022.01.001
[69] Mikkelsen, Robert L. “Managing Potassium for Organic Crop Production”. HortTechnology, vol 17, issue 4, Oct-Dec 2007. PDF. https://journals.ashs.org/downloadpdf/view/journals/horttech/17/4/article-p455.pdf
[70] Sideman, Eric. “Potassium”. Fall 2011. Maine Organic Farmers and Gardeners. Website. Accessed June 28, 2024. https://www.mofga.org/resources/soil/potassium/
[71] Sean. “How to use a Magnesium Fire Starter” Jan 28, 2023. Explore Camping Life. Website. Accessed Apr 8, 2024. https://explorecampinglife.com/how-to-use-a-magnesium-fire-starter/
- These are tools for survival situations, not toys. Because of climate change, the dangers of forest fires are higher than ever before. One mistake with a fire starter could kill innocent people and destroy their belongings.
[72] Kleczkowski, Leszek A. Igamberdlev, Abir U. “Magnesium Signaling in Plants”. Feb 2021. Int J Mol Sci, vol. 22, no. 3, p.1159. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7865908/
[73] Chairiyah, N. Harijati, N. Mastuti, R. “Variation of Calcium Oxalate (CaOx) Crystals in Porang Corms (Amorphophallus muelleri Blume) at Different Harvest Time”. Feb 2016. American Journal of Plant Sciences, vol. 7, no. 2. https://doi.org/10.4236/ajps.2016.72030
[74] Franceschi, Vincent. “Calcium oxalate in plants”. Jul 1, 2001. Trends in Plant Science. Cell, vol. 6, iss. 7, p.331. Accessed Mar 27, 2024. https://doi.org/10.1016/S1360-1385(01)02014-3
[75] Williams, Mary. “Review: Illuminating the hidden world of calcium ions in plants with a universe of indicators (Plant Physiol)”. Oct 8, 2021. Wikimedia Commons. Accessed Mar 15, 2024. https://plantae.org/review-illuminating-the-hidden-world-of-calcium-ions-in-plants-with-a-universe-of-indicators-plant-physiol/
[76] Hedrich, Rainer. Kudla, Jörg. “Calcium Signaling Networks Channel Plant K+ Uptake”. Jun 30, 2006. Vol 125, Iss. 7, p.1221-1223. CellPress Journal. https://www.cell.com/fulltext/S0092-8674(06)00775-6#figures
[77] Kinjo, Tashi G. Schnetkamp, Paul PM. “Ca2+ Chemistry, Storage and Transport in Biologic Systems: An Overview”. Channels and Transporters. Madame Curie Bioscience Database. Landes Bioscience. National Center for Biotechnology Information (NCBI). National Library of Medicine (NLM). National Institutes of Health (NIH). Accessed Mar 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK5959/
[78] Moore, J. Zhou, J. Garand, E. “Basics of Organic Nomenclature”. Appendix. Chemistry 109, Fall 2020. UW-Madison. Pressbooks. Unizin. Accessed Mar 27, 2024. https://wisc.pb.unizin.org/chem109fall2020ver03/
[79] Lautner, S. et al. “Calcium nutrition has a significant influence on wood formation in poplar”. Jan 8, 2007. New Phytologist, vol. 173, iss. 4, pp.743-752. Accessed Mar 27, 2024. https://nph.onlinelibrary.wiley.com/doi/10.1111/j.1469-8137.2007.01972.x
[80] Takahashi, H. Marsolais, F. Cuypers, A. Kopriva, S. “Sulfur metabolism: actions for plant resilience and environmental adaptation”. Jun 6, 2023. J Exp Bot, vol. 74, no. 11, pp.3271-3275. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10243964/
[81] “Melting and Pouring Metal”. Reliance Foundry. Website. Accessed Mar 28, 2024. https://www.reliance-foundry.com/blog/melting-metal-pouring
[82] Mydy, LS. Chigumba, DN. Kersten, RD. “Plant Copper Metalloenzymes As Prospects for New Metabolism Involving Aromatic Compounds”. Nov 29, 2021 vol 12, no. 692108. Front Plant Sci. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8672867/
[83] Wairich, et al. “Throwing Copper Around: How Plants Control Uptake, Distribution, and Accumulation of Copper”. Apr 21, 2022. Agronomy 2022, vol. 12, iss. 5, p.994. https://doi.org/10.3390/agronomy12050994
[84] “ES6120006”. Natura 2000 – Standard Data Form. Natura2000. European Union (EU). Website. Accessed Apr 4, 2024. http://natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=ES6120006
[85] “USDA Organic”. USDA. Accessed Mar 28, 2024. https://www.usda.gov/topics/organic
[86] “Using non-organic ingredients”. Oregon Tilth. Website. Accessed Mar 28, 2024. https://tilth.org/help-center/using-non-organic-ingredients/
[87] “§ 205.601 Synthetic substances allowed for use in organic crop production.”. 7 CFR Part 205 Subpart G. Code of Federal Regulations (eCFR). Accessed Mar 28, 2024. https://www.ecfr.gov/current/title-7/subtitle-B/chapter-I/subchapter-M/part-205/subpart-G
[88] Cohrssen, John J. Miller, Henry I. “The USDA’s Meaningless Organic Label”. Spring 2016. Regulation. Cato Institute. Accessed Mar 28, 2024. https://www.cato.org/regulation/spring-2016/usdas-meaningless-organic-label
[89] “Mercury Emissions: The Global Context”. Apr 25, 2023. Environmental Protection Agency (EPA). Accessed Feb 15, 2024. https://www.epa.gov/international-cooperation/mercury-emissions-global-context
[90] Bishop, K. Nilsson, MB. “The health threat from mercury in freshwater fish could be blowing away in the wind”. Nov 22, 2017. Swedish University of Agricultural Sciences. Accessed Feb 15, 2024. https://www.slu.se/en/ew-news/2017/11/the-health-threat-from-mercury-in-freshwater-fish-could-be-blowing-away-in-the-wind/
[91] Tenenbaum, David. “Maps showing potential for soil contamination issued for Wisconsin’s lead-zinc mining district”. Oct 7, 2019. UW-Madison News. Accessed June 28, 2024. https://news.wisc.edu/maps-showing-potential-for-soil-contamination-issued-for-wisconsins-lead-zinc-mining-district/
- See maps of natural occurrence of Lead (Pb) contamination.
[92] “Lead-Safe Wisconsin: Universal Testing”. Wisconsin Department of Health Services. Website. Accessed June 28, 2024. https://www.dhs.wisconsin.gov/lead/universal-testing.htm
[93] Tenenbaum, David. “New method assesses lead hazard in soil”. Jan 18, 2019. UW-Madison News. Accessed June 28, 2024. https://news.wisc.edu/new-method-assesses-lead-hazard-in-soil/
[94] Sussman, Mary. “Lead exposure increases in Milwaukee during pandemic”. Oct 28, 2020. Shepherd Express. University of Wisconsin – Milwaukee (UWM). https://uwm.edu/publichealth/lead-exposure-increases-in-milwaukee-during-pandemic/
[95] Swarns, Rachel L. “Lead Found in White House Garden”. July 2, 2009. NYTimes. Accessed June 28, 2024. https://archive.nytimes.com/thecaucus.blogs.nytimes.com/2009/07/02/lead-found-in-white-house-garden/
[96] “White House Unveils New Lead Removal Initiatives”. Jan 31, 2023. PaintSquare. Accessed June 28, 2024. https://www.paintsquare.com/news/view/?26033
[97] Dopico, Miguel. Gómez, Alberto. “Review of the current state and main sources of
dioxins around the world”. Aug 15, 2015. Journal of the Air & Waste Management Association, vol 65, issue 9, pp. 1033-1049. https://doi.org/10.1080/10962247.2015.1058869
[98] “Learn about Dioxin”. United States Environmental Protective Agency (EPA). Accessed July 2, 2024. https://www.epa.gov/dioxin/learn-about-dioxin
[99] “Environmental Remediation of Dioxin Contamination at Danang Airport Project”. USAID. Accessed July 2, 2024. https://www.usaid.gov/vietnam/fact-sheets/environmental-remediation-dioxin-contamination-danang-airport-project
[100] “Danang Remediation Process”. USAID. Accessed July 2, 2024. https://www.usaid.gov/vietnam/danang-remediation-process
[101] USAIDVietnam. “In-Pile Thermal Desorption (IPTD) Animation”. May 6, 2014. YouTube. Accessed July 2, 2024. https://www.youtube.com/watch?v=HChULmd9eOk
[102] Littlejohn, Ronnie. “Daoist Philosophy”. Internet Encyclopedia of Philosophy (IEP). Website. Accessed Apr 4, 2024. https://iep.utm.edu/daoismdaoist-philosophy/
- Of interest, is the concept of Wu Wei (無為) and its relationship to the quality of action. It is included as a cultural inclusive and philosophical resource, and not as a promotion for a religion.
Images
Featured Image: Tigran Mitr am. “File:Gray forest soil.JPG”. Jun 25, 2009. Wikimedia Commons. Accessed Mar 13, 2024. https://commons.wikimedia.org/wiki/File:Gray_forest_soil.JPG
[1a] Mohr, Jens. “File:Kommandostav kikare, 1610-1632 – Livrustkammaren – 106404.tif”. Swedish Royal Armoury. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:Kommandostav_kikare,1610-1632–Livrustkammaren-_106404.tif
[…] Mohr, Jens. “File:Kommandostav-kikare, 1610-1632 – Livrustkammaren – 106405.tif”. 1610-1632. Swedish Royal Armoury. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:Kommandostav-kikare,1610-1632–Livrustkammaren-_106405.tif
[2a] Galilei, Galileo. “File:Galileo Galilei, Disegni originali delle Lune, 1609, Biblioteca Nazionale Centrale, Firenze.jpg”. Oct 1, 2008. Biblioteca Nazionale, Firenze, Italia. Wikimedia Commons. Accessed Feb 01, 2024. https://commons.wikimedia.org/wiki/File:Galileo_Galilei,_Disegni_originali_delle_Lune,_1609,_Biblioteca_Nazionale_Centrale,_Firenze.jpg
[3a] Mysid. “File:Atmospheric electromagnetic opacity.svg”. Dec 27, 2008. NASA. Wikimedia Commons. Accessed Aug 7, 2024. https://commons.wikimedia.org/wiki/File:Atmospheric_electromagnetic_opacity.svg
[4a] DRJacobson. “File:Atacama Submillimeter Telescope Experiment complex, Chile.jpg”. Jul 6, 2008. Wikimedia Commons. Accessed Aug 7, 2024. https://en.wikipedia.org/wiki/File:Atacama_Submillimeter_Telescope_Experiment_complex,_Chile.jpg
[5a] Smoot’s research group. “File:Bersanelli-smoot.jpg”. May 12, 2009. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:Bersanelli-smoot.jpg
[…] Peel, Mike. “File:Model of the Planck Satellite.jpg”. Apr 20, 2009. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:Model_of_the_Planck_Satellite.jpg
[6a] “Planck’s view of the cosmic microwave background”. June 17, 2018. European Space Agency (ESA). Planck Institute. Accessed Dec 17, 2024. https://www.esa.int/Science_Exploration/Space_Science/Planck/Planck_and_the_cosmic_microwave_background
[7a] “File:CMB Timeline75.jpg”. NASA/WMAP Science Team. Wikimedia Commons. Accessed Dec 17, 2024. https://commons.wikimedia.org/wiki/File:CMB_Timeline75.jpg
[8a] Macrobius Ambrosius Theodosius. “File:Nks218 101.jpg”. 1150. Wikimedia Commons. Accessed Feb 12, 2024. https://commons.wikimedia.org/wiki/File:Nks218_101.jpg
[…] Petrus Apianus. “File:Astronomicum Caesareum (1540).f23.jpg”. 1540. Wikimedia Commons. Accessed Feb 12, 2024. https://commons.wikimedia.org/wiki/File:Astronomicum_Caesareum_(1540).f23.jpg
[9a] al-Biruni. “File:Lunar phases al-Biruni.jpg”. ~1000AD. Wikimedia Commons. Accessed Dec 1, 2023. https://commons.wikimedia.org/wiki/File:Lunar_phases_al-Biruni.jpg
[10a] Sacro Bosco, Joannes de. “De sphaera, [Text 5]”. 1240-1260. The New York Public Library (NYPL) Digital Collections. Accessed Dec 1, 2023. https://digitalcollections.nypl.org/items/510d47da-e573-a3d9-e040-e00a18064a99#/?uuid=0c555150-f054-0138-2661-0242ac110003&rotate=0
[11a] “File:Ilustração eclipse solar de 1858.jpg”. Sept 7, 1858. Brazilian National Archives. Wikimedia Commons. Accessed Dec 1, 2023. https://commons.wikimedia.org/wiki/File:Ilustra%C3%A7%C3%A3o_eclipse_solar_de_1858.jpg
[12a] “File:Magnificent CME Erupts on the Sun with Earth to Scale.jpg”. Sept 5, 2012. NASA Goddard Space Flight Center. Wikimedia Commons. Accessed Nov 1, 2023. https://commons.wikimedia.org/wiki/File:Magnificent_CME_Erupts_on_the_Sun_with_Earth_to_Scale.jpg
[13a] Lasmpascal. “File:Planets and sun size comparison.jpg”. Jan 23, 2012. Wikimedia Commons. Accessed Dec 1, 2023. https://commons.wikimedia.org/wiki/File:Planets_and_sun_size_comparison.jpg
[14a] Someone. “File:Nuclear fusion.gif”. Nov 3, 2011. Wikimedia Commons. Accessed Feb 8, 2024. https://commons.wikimedia.org/wiki/File:Nuclear_fusion.gif
[…] MikeRun. “File:Nuclear fission reaction.svg”. Jun 25, 2017. Wikimedia Commons. Accessed Feb 8, 2024. https://commons.wikimedia.org/wiki/File:Nuclear_fission_reaction.svg
[15a] Helebrant, Jan.”File:GDCN0024216 banded iron ore (50189012776).jpg”. Aug 4, 2020. Wikimedia Commons. Accessed Feb 02, 2023. https://commons.wikimedia.org/wiki/File:GDCN0024216_banded_iron_ore_(50189012776).jpg
[…] Helebrant, Jan. “File:GDCN0024214 banded iron ore (50189010436).jpg”. Aug 4, 2020. Wikimedia Commons. Accessed Feb 02, 2023. https://commons.wikimedia.org/wiki/File:GDCN0024214_banded_iron_ore_(50189010436).jpg
[…] “File:Magnetic reconnection zones in the earth’s magnetosphere Science 1 th-jpg.jpg”. Dec 18, 2023. NASA. Wikimedia Commons. Accessed Feb 02, 2024. https://commons.wikimedia.org/wiki/File:Magnetic_reconnection_zones_in_the_earth%27s_magnetosphere_Science_1_th-jpg.jpg
[…] Glatzmaier, Gary A. “File:Geodynamo Between Reversals.gif”. Dec 29, 2003. Wikimedia Commons. Accessed Feb 02, 2024. https://commons.wikimedia.org/wiki/File:Geodynamo_Between_Reversals.gif
[16a] “File:Ocean world Earth.jpg”. Jul 17, 1969. Wikimedia Commons. Accessed Mar 4, 2024. https://commons.wikimedia.org/wiki/File:Ocean_world_Earth.jpg
[17a] Woudloper. “File:Geologic map Wales & SW England EN.svg”. Nov 2009. Wikimedia Commons. Accessed Mar 13, 2024. https://commons.wikimedia.org/wiki/File:Geologic_map_Wales_%26_SW_England_EN.svg
[…] “Figure 2”. The Soil, 31. Soil and Plant Nutrition. Apr 16, 2018. OpenStax Biology, 2nd Edition. Accessed Mar 13, 2024. https://courses.lumenlearning.com/suny-osbiology2e/chapter/the-soil/
[18a] “Rock Cycle”. Siyavula Education. “3.5 THE ROCK CYCLE”. Dastrup, AR. “Physical Geography and Natural Disasters”. Salt Lake Community College. Accessed Mar 13, 2024. https://slcc.pressbooks.pub/physicalgeography/chapter/3-5/
[19a] Clamosa, Fama. “File:Gondwana 420 Ma.png”. GPlates. March 3, 2018. Wikimedia Commons. Accessed Nov 27, 2023. https://commons.wikimedia.org/wiki/File:Gondwana_420_Ma.png
[20a] Holland Baptista, Dalton. “File:Sandstone cliffs close to Analandia.jpg”. 2009. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Sandstone_cliffs_close_to_Analandia.jpg
- Overlayed image exported from OpenStreetMap, map of Village Analandia, Brazil.
[21a] St. John, James. “Spirifer invalidistriatus (fossil brachiopods) (Byer Sandstone, Lower Mississippian; Black Hand Gorge, Ohio, USA) 1”. Nov 22, 2017. Flickr. Accessed Mar 5, 2024. https://www.flickr.com/photos/jsjgeology/37931683764/in/photostream/
[…] St. John, James. “File:Byer Sandstone (Lower Mississippian; State Farm Quarry, Newark, Ohio, USA) 3 (32387546360).jpg”. Feb 1, 2017. Wikimedia Commons. Accessed Feb 4, 2024. https://commons.wikimedia.org/wiki/File:Byer_Sandstone_(Lower_Mississippian;State_Farm_Quarry,_Newark,_Ohio,_USA)_3(32387546360).jpg
- Overlayed image exported from OpenStreetMap, map of Newark, Ohio, USA.
[22a] Sederholm, J. “File:Valun-sedergolm-1911.png”. 1911. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Valun-sedergolm-1911.png
[…] Stockner, Clemens. “File:Mittivakkat edge 25.jpg”. Jul 30, 2016. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Mittivakkat_edge_25.jpg
- Overlayed image exported from OpenStreetMap, map of Mittivakkat Glacier, Greenland, from Wikipedia article’s GeoHack coordinates.
[23a] Mroczek, Przemysław. “File:Tyszowce loess section.jpg”. May 20, 2002. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Tyszowce_loess_section.jpg
- Overlayed image exported from OpenStreetMap, map of Tyszowce, Poland.
[24a] Bunk, S. “File:Northwest Crown Fire Experiment.png”. Feb 17, 2004. USDA Forest Service. Wikimedia Commons. Accessed June 30, 2024. https://commons.wikimedia.org/wiki/File:Northwest_Crown_Fire_Experiment.png
[…] Jynto. Rohde, RA. Jacek, FH. “File:Combustion reaction of methane.jpg”. March 2, 2013. Wikimedia Commons. Accessed June 30, 2024. https://commons.wikimedia.org/wiki/File:Combustion_reaction_of_methane.jpg
[…] MikeRun. “File:Burning-wood-on-scale.svg”. Aug 27, 2021. Wikimedia Commons. Accessed June 30, 2024. https://commons.wikimedia.org/wiki/File:Burning-wood-on-scale.svg
[25a] Rozenberg, Avigail. “File:PikiWiki Israel 14474 Wildlife and Plants of Israel-01.jpg”. Sept 28, 2010. Wikimedia Commons. Accessed Feb 15, 2024. https://commons.wikimedia.org/wiki/File:PikiWiki_Israel_14474_Wildlife_and_Plants_of_Israel-01.jpg
[…] Vassil. “File:Paris Concorde obélisque 2.jpg”. May 14, 2007. Wikimedia Commons. Accessed Feb 15, 2024. https://commons.wikimedia.org/wiki/File:Paris_Concorde_ob%C3%A9lisque_2.jpg
[…] Dahl, Jeff. “File:Edwin Smith Papyrus v2.jpg”. 1600BC. Wikimedia Commons. Accessed Feb 15, 2024. https://commons.wikimedia.org/wiki/File:Edwin_Smith_Papyrus_v2.jpg
[…] Gamal, Sandy. “File:Egyptian Hieroglyphs.jpg”. Apr 25, 2023. Wikimedia Commons. Accessed Feb 15, 2024. https://commons.wikimedia.org/wiki/File:Egyptian_Hieroglyphs.jpg
[…] akhenatenator. “File:Day 1 – Shabtis (8145939981).jpg”. Nov 1, 2012. Wikimedia Commons. Accessed Feb 15, 2024. https://commons.wikimedia.org/wiki/File:Day_1_-Shabtis(8145939981).jpg
[…] Theasby, Neil. “File:Brown sheep at Brindwoodgate – geograph.org.uk – 6176998.jpg”. Jun 9, 2019. Wikimedia Commons. Accessed Jul 5, 2024. https://commons.wikimedia.org/wiki/File:Brown_sheep_at_Brindwoodgate_-_geograph.org.uk_-_6176998.jpg
[26a] 魏伯阳 (Wei Boyang). “File:NAJDA-311-0272 周易参同契 下.pdf”. 周易参同契 下 (Zhouyi Cantong Qi, Part 2). National Archives of Japan. Wikimedia Commons. Accessed Dec 17, 2024. https://commons.wikimedia.org/w/index.php?title=File:NAJDA-311-0272_%E5%91%A8%E6%98%93%E5%8F%82%E5%90%8C%E5%A5%91_%E4%B8%8B.pdf&page=16
[27a] Jabir ibn Hayyan. “File:Drawing and description of Alembic , by Jabir Ibn Hayyan in 8th century.jpg”. Jan 1, 776AD. Wikimedia Commons. Accessed Dec 1, 2023. https://commons.wikimedia.org/wiki/File:Drawing_and_description_of_Alembic_,_by_Jabir_Ibn_Hayyan_in_8th_century.jpg
[28a] “File:Mendeleev Photographische Gesellschaft.jpg”. ~1910. Photographische Gesellschaft, Berlin. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:Mendeleev_Photographische_Gesellschaft.jpg
[…] Aljuh. “File:Mendeleev mauscrit original.jpg”. Apr 9, 2017. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:Mendeleev_mauscrit_original.jpg
[29a] Kurzon. “File:Geiger-Marsden experiment.svg”. Apr 15, 2014. Wikimedia Commons. Accessed Dec 17, 2024. https://commons.wikimedia.org/wiki/File:Geiger-Marsden_experiment.svg
[…] Hernitschek, Nina. “File:Apfel partikel.jpg”. Nov 26, 2002. Wikimedia Commons. Accessed Nov 23, 2023. https://commons.wikimedia.org/wiki/File:Apfel_partikel.jpg
[30a] Gesing, Thorsten M. Uecker, Reinhard. Buhl, J.-Christian. “Refinement of the crystal structure of praseodymium orthoscandate, PrScO3“. Zeitschrift für Kristallographie – New Crystal Structures, vol. 224, no.3, 2009, pp.365-366. https://doi.org/10.1524/ncrs.2009.0159
[…] Zhen Chen et al., “Electron ptychography achieves atomic-resolution limits set by lattice vibrations”. Science, vol. 372, pp.826-831, 2021. https://doi.org/10.1126/science.abg2533
[31a] Harrison, JJ. “File:Quartz, Tibet.jpg”. Feb 2, 2009. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Quartz,_Tibet.jpg
[…] Géry, Parent. “File:Quartz rose (Brésil) 1.JPG”. Jun 21, 2011. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Quartz_rose_(Br%C3%A9sil)_1.JPG
[…] Andel. “File:Α-Quartz.svg”. Jun 17, 2019. Accessed Mar 5, 2024. https://en.wikipedia.org/wiki/File:%CE%91-Quartz.svg
[…] Andel. “File:Β-Quartz.svg”. Jun 17, 2019. Accessed Mar 5, 2024. https://en.wikipedia.org/wiki/File:%CE%92-Quartz.svg
[…] Lavinsky, Robert M. “File:Mimetite-Quartz-290590.jpg”. March 2010. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Mimetite-Quartz-290590.jpg
[…] Sonitron Support. “File:Piezo bending principle.svg”. Feb 17, 2022. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Piezo_bending_principle.svg
[32a] KES47. “File:Rainbow1.svg”. Jun 4, 2010. Wikimedia Commons. Accessed Dec 15, 2023. https://commons.wikimedia.org/wiki/File:Rainbow1.svg
[…] Karasik, Nikita. “File:Rainbow optical effect.JPG”. Sept 5, 2012. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Rainbow_optical_effect.JPG
[33a] Fröbel, Friedlich. “File:Chlorophyll Dünnschichtchromatographie.jpg”. Sept 28, 2011. Wikimedia Commons. Accessed Dec 15, 2023. https://commons.wikimedia.org/wiki/File:Chlorophyll_D%C3%BCnnschichtchromatographie.jpg
[34a] Offnfopt. “File:Gas chromatograph-vector.svg”. Apr 10, 2015. Wikimedia Commons. Accessed Dec 15, 2023. https://commons.wikimedia.org/wiki/File:Gas_chromatograph-vector.svg
[…] Lavitt, Alan. “File:Female scientist working with gas chromatograph.jpg”. Wikimedia Commons. Accessed Dec 15, 2023. https://commons.wikimedia.org/wiki/File:Female_scientist_working_with_gas_chromatograph.jpg
[35a] “File:Empedocles four elements.jpg”. Dec 16, 2018. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Empedocles_four_elements.jpg
[…] von Leibniz, Gottfried Wilhelm. “File:Leibniz four elements.jpg”. Apr 22, 2015. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Leibniz_four_elements.jpg
[…] Cohen, Tobias. “File:Diagram of four elements, 18th century in Tobias Cohen Wellcome L0015958.jpg”. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Diagram_of_four_elements,_18th_century_in_Tobias_Cohen_Wellcome_L0015958.jpg
[…] Teepetersen. “File:Four Elements.jpg”. Oct 1, 2014. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Four_Elements.jpg
[…] Dalton, John. “File:John Dalton. Symbols representing chemical elements Wellcome L0007206.jpg”. Wikimedia Commons. Accessed Nov 23, 2023. https://commons.wikimedia.org/wiki/File:John_Dalton._Symbols_representing_chemical_elements_Wellcome_L0007206.jpg
[36a] “File:Wide Periodic Table (corrected).svg”. Jan 28, 2012. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Wide_Periodic_Table_(corrected).svg
[37a] Ingalls, Bill. “File:Artemis I Prelaunch (NHQ202208310014).jpg”. Aug 31, 2022. Wikimedia Commons. Accessed Feb 26, 2024. https://commons.wikimedia.org/wiki/File:Artemis_I_Prelaunch_(NHQ202208310014).jpg
[38a] MikeRun. “File:Hydrogen-atom-electron-jump-examples.svg”. Mar 4, 2021. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:Hydrogen-atom-electron-jump-examples.svg
[39a] Bautsch. “File:Spektrum.Balmer-Serie.png” Apr 1, 2021. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:Spektrum.Balmer-Serie.png
[40a] Geek3. “File:Atomic orbitals n1234 m-eigenstates.png”. Apr 1, 2018. Wikimedia Commons. Accessed Dec 2, 2023. https://commons.wikimedia.org/wiki/File:Atomic_orbitals_n1234_m-eigenstates.png
[41a] FischX. “File:Carbon cycle-simple diagram.svg”. Wikimedia Commons. Accessed Aug 5, 2024. https://commons.wikimedia.org/wiki/File:Carbon_cycle-simple_diagram.svg
[42a] “File:Atmosfaerisk spredning.png”, Wikimedia Commons, https://upload.wikimedia.org/wikipedia/commons/1/1c/Atmosfaerisk_spredning.png
[43a] ILoveAllTrees. “File:Anthracite (coal).jpg”. May 7, 2022. Wikimedia Commons. Accessed Feb 26, 2024. https://commons.wikimedia.org/wiki/File:Anthracite_(coal).jpg
[…] Delluc. “File:B. et G. Delluc à Lascaux.jpg”. Jul 13, 2011. Wikimedia Commons. Accessed Feb 26, 2024. https://commons.wikimedia.org/wiki/File:B._et_G._Delluc_%C3%A0_Lascaux.jpg
[…] Bendo, Dati. “File:Charcoal 8.jpg”. Aug 19, 2020. Wikimedia Commons. Accessed Feb 26, 2024. https://commons.wikimedia.org/wiki/File:Charcoal_8.jpg
[…] Romary. “File:Charbon de bois rouge.jpg”. April 2007. Wikimedia Commons. Accessed Feb 26, 2024. https://commons.wikimedia.org/wiki/File:Charbon_de_bois_rouge.jpg
[44a] Samsiq. “File:Direct visualization of valence and core electrons in carbon.png”. Jun 25, 2021. Wikimedia Commons. Accessed Dec 5, 2023. https://commons.wikimedia.org/wiki/File:Direct_visualization_of_valence_and_core_electrons_in_carbon.png
[…] Pumbaa. Robson, Greg. “File:Electron shell 006 Carbon – no label.svg”. Wikimedia Commons. Accessed Dec 5, 2023. https://commons.wikimedia.org/wiki/File:Electron_shell_006_Carbon_-_no_label.svg
[…] SM358. “File:Carbon-125.svg” Nov 15, 2021. Wikimedia Commons. Accessed Dec 5, 2023. https://commons.wikimedia.org/wiki/File:Carbon-125.svg
[45a] “BSCI 1510L Literature and Stats Guide: C3 and C4 Plants”. Research Guides. Vanderbilt University. Accessed Dec 5, 2023. https://researchguides.library.vanderbilt.edu/c.php?g=69346&p=809936
[46a] Koshravesh, R. Stata, M. Adachi, S. Sage, TL. Sage, RF. “Evolutionary Convergence of C4 Photosynthesis: A Case Study in Nyctaginaceae”. Nov 01, 2020. Frontiers in Plant Science. Hypothesis and Theory, vol. 11, 2020. https://doi.org/10.3389/fpls.2020.578739
[47a] Clayton, Michael W. “Cross section through a water lily leaf”. UW-Madison Digitized Collections (UWDC). Accessed Dec 7, 2023. https://digital.library.wisc.edu/1711.dl/2ARVK6FYLW5PA86
[48a] Glover, Nika. “File:Liquid oxygen in a beaker.jpg”. Oct 14, 2010. Wikimedia Commons. Accessed Feb 26, 2024. https://commons.wikimedia.org/wiki/File:Liquid_oxygen_in_a_beaker.jpg
[…]
[49a] Trazyanderson. “File:Water Strider, Rush River, Wisconsin.jpg”. Jul 15, 2009. Wikimedia Commons. Accessed Dec 15, 2023. https://commons.wikimedia.org/wiki/File:Water_Strider,_Rush_River,_Wisconsin.jpg
[…] Zimbres, Eurico. Booyabazooka. “File:Water molecule as eletric dipole.png”. Apr 28, 2006. Wikimedia Commons. Accessed Dec 15, 2023. https://commons.wikimedia.org/wiki/File:Water_molecule_as_eletric_dipole.png
[50a] MichelleLily14. “File:Auberginen gesalzen.jpg”. Jul 31, 2018. Wikimedia Commons. Accessed Dec 15, 2023. https://commons.wikimedia.org/wiki/File:Auberginen_gesalzen.jpg
[…] OpenStax. “File:0307 Osmosis.jpg”. May 18, 2016. Wikimedia Commons. Accessed Dec 15, 2023. https://commons.wikimedia.org/wiki/File:0307_Osmosis.jpg
[…] Kelvinsong. “File:Into the stele.svg”. Jul 31, 2018. Wikimedia Commons. Accessed Dec 15, 2023. https://commons.wikimedia.org/wiki/File:Into_the_stele.svg
[51a] Lou Gold. “plant evapotranspiration in Rio Branco”. Apr 7, 2010. Flickr. Accessed Dec 15, 2023. https://www.flickr.com/photos/visionshare/4500284946/
[…] BlueRidgeKitties. “Leaf Cross-Section”. Dec 8, 2011. Wikimedia Commons. Accessed Dec 15, 2023. https://www.flickr.com/photos/blueridgekitties/6475212645
[52a] van der Sleen, Wicher Gosen Nicolaas. “File:COLLECTIE TROPENMUSEUM Oogst van suikerriet dat verwerkt zal worden in de suikerfabriek Gesiekan bij Jogjakarta TMnr 10028320.jpg”. Oct 7, 1929. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:COLLECTIE_TROPENMUSEUM_Oogst_van_suikerriet_dat_verwerkt_zal_worden_in_de_suikerfabriek_Gesiekan_bij_Jogjakarta_TMnr_10028320.jpg
[…] Collins, Marjory. “File:Mexican agricultural laborer topping sugar beets8d29109v.jpg”. May 1943. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Mexican_agricultural_laborer_topping_sugar_beets8d29109v.jpg
[…] Lionceau, Joseph. “File:Women fanning Sorghum.png”. Apr 5, 2022. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Women_fanning_Sorghum.png
[53a] Caesar, Norman. “File:Hawthorn tree growing on rock – geograph.org.uk – 3732148.jpg”. Nov 4, 2013. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Hawthorn_tree_growing_on_rock_-_geograph.org.uk_-_3732148.jpg
[54a] Harris, Trevor. “File:Soil profile – geograph.org.uk – 3114127.jpg”. Aug 13, 2012. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Soil_profile_-_geograph.org.uk_-_3114127.jpg
[…] McLund. “File:Soil profile 0-125cm.jpg”. Jul 15, 2021. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Soil_profile_0-125cm.jpg
[…] Halling, Philip. “File:Soil profile in forest above nant Rhyd-wen – geograph.org.uk – 1425702.jpg”. Aug 1, 2009. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Soil_profile_in_forest_above_nant_Rhyd-wen_-_geograph.org.uk_-_1425702.jpg
[…] Nikanos. “File:Bodenprofil Heide.jpg”. Oct 4, 2006. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Bodenprofil_Heide.jpg
[…] Gnangarra. “File:Perth basin soil profile gnangarra-19.jpg”. May 29, 2012. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Perth_basin_soil_profile_gnangarra-19.jpg
[…] Stewart, Ailith. “File:Podzol – geograph.org.uk – 218892.jpg”. Jul 20, 2006. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Podzol_-_geograph.org.uk_-_218892.jpg
[…] Remarquemo. “File:Podzolization in Podzol soils.png”. May 4, 2018. Wikimedia Commons. Accessed Feb 28, 2024. https://commons.wikimedia.org/wiki/File:Podzolization_in_Podzol_soils.png
[55a] Guffogg, Julian P. “File:Fertile Lincolnshire soil – geograph.org.uk – 3699381.jpg”. Oct 13, 2013. Geograph Britain and Ireland. Wikimedia Commons. Accessed Jul 2, 2024. https://commons.wikimedia.org/wiki/File:Fertile_Lincolnshire_soil_-_geograph.org.uk_-_3699381.jpg
[…] Hodnett, Ryan. “File:Peat Moss (Sphagnum sp.) – London, Ontario 2015-04-12 (02).jpg”. Apr 12, 2015. Wikimedia Commons. Accessed Jul 2, 2024. https://commons.wikimedia.org/wiki/File:Peat_Moss_(Sphagnum_sp.)_-_London,_Ontario_2015-04-12_(02).jpg
[…] Ragesoss. “File:Schultz Sphagnum Peat Moss.jpg”. Jul 6, 2008. Wikimedia Commons. Accessed Jul 2, 2024. https://commons.wikimedia.org/wiki/File:Schultz_Sphagnum_Peat_Moss.jpg
[…] Phương Huy. “File:Chậu mầm cây kiểng (xương rồng Việt Nam) năm 2016 (1).JPG”. 2016. Wikimedia Commons. Accessed Jul 2, 2024. https://commons.wikimedia.org/wiki/File:Ch%E1%BA%ADu_m%E1%BA%A7m_c%C3%A2y_ki%E1%BB%83ng_(x%C6%B0%C6%A1ng_r%E1%BB%93ng_Vi%E1%BB%87t_Nam)_n%C4%83m_2016_(1).JPG
[56a] “File:Tulsky Greenhouse Complex 04.jpg”. Oct 27, 2020. Пресс-служба губернатора Тульской области (Govenor of Tula Region’s Press Service). Wikimedia Commons. Accessed Jul 2, 2024. https://commons.wikimedia.org/wiki/File:Tulsky_Greenhouse_Complex_04.jpg
[…] Joi Ito. “File:Strawberry greenhouse.jpg”. Feb 16, 2008. Wikimedia Commons. Accessed Jul 2, 2024. https://commons.wikimedia.org/wiki/File:Strawberry_greenhouse.jpg
[…] Starr, Forest. Starr, Kim. “File:Starr-100623-7801-Ocimum basilicum-potted plants in greenhouse-Pukalani Plant Company Pulehu-Maui (24746810490).jpg”. Jun 23, 2010. Wikimedia Commons. Accessed Jul 2, 2024. https://commons.wikimedia.org/wiki/File:Starr-100623-7801-Ocimum_basilicum-potted_plants_in_greenhouse-Pukalani_Plant_Company_Pulehu-Maui_(24746810490).jpg
[…] Gian Waru. “File:Propagation.jpg”. Nov 23, 2007. Wikimedia Commons. Accessed Jul 2, 2024. https://commons.wikimedia.org/wiki/File:Propagation.jpg
[57a] McKee, Edwin Dinwiddie. “Scanning electron microscope photograph of Supai Group clay minerals …”. 1982. United States Geological Survey (USGS). Accessed Feb 01, 2024. https://www.sciencebase.gov/catalog/item/51dd8a57e4b0f72b4471c260
[…] “File:Sand under electron microscope.jpg”. NASA. Wikimedia Commons. Accessed Feb 1, 2024. https://commons.wikimedia.org/wiki/File:Sand_under_electron_microscope.jpg
- Ottowa Silica Sand, an industrial by-product from hydraulic mining of St. Peter Sandstone in Illinois.
[…] St. John, James. “File:Marine biogenous aragonite sand (modern; sandy beach at French Bay, San Salvador Island, Bahamas) (48739969587).jpg”. Sep 15, 2019. Wikimedia Commons. Accessed Feb 1, 2024. https://commons.wikimedia.org/wiki/File:Marine_biogenous_aragonite_sand_(modern;_sandy_beach_at_French_Bay,_San_Salvador_Island,_Bahamas)_(48739969587).jpg
[58a] “File:Crops NR 37 (38808783752).jpg”. Jul 19, 2012. USDA NRCS. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Crops_NR_37_(38808783752).jpg
[…] White, James Francis. “File:Tetrads of bacterium Micrococcus luteus in root cells of plant Rumex crispus.jpg”. Oct 26, 2017. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Tetrads_of_bacterium_Micrococcus_luteus_in_root_cells_of_plant_Rumex_crispus.jpg
[…] Agne27. “File:Downy and Powdery mildew on grape leaf.JPG”. USDA ARS. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Downy_and_Powdery_mildew_on_grape_leaf.JPG
[59a] “Gartenkresse Erwebsanbau”. Hortipendium.de. Accessed May 6th, 2024. https://hortipendium.de/images/thumb/1/1c/Minimum-Tonne.svg/660px-Minimum-Tonne.svg.png
[…] “Diagrama de Mulder”. Homo agricola. Blogspot. Accessed May 6th, 2024. http://elhocino-adra.blogspot.com/2015/03/diagrama-de-mulder.html
[60a] Ackerman, Sarah. “File:Making Liquid Nitrogen Ice Cream – Sentosa, Singapore – 16 Oct. 2013.jpg”. Oct 16, 2013. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Making_Liquid_Nitrogen_Ice_Cream_-Sentosa,_Singapore-_16_Oct._2013.jpg
[…] Goetzinger, Benjamin. “File:Chile – Humberstone – Salpeter – sodium nitrate.jpg”. Aug 26, 2010. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Chile_-Humberstone–Salpeter-_sodium_nitrate.jpg
[…] Shafee, Thomas. “File:Alpha beta structure (full).png”. Feb 12, 2017. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Alpha_beta_structure_(full).png
[61a] Mohatatou. “File:Earth’s atmosphere Tunisia 2019.jpg”. Jun 30, 2019. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Earth%27s_atmosphere_Tunisia_2019.jpg
[…] Loxton, Sharon. “File:Severnside fertilizer works – geograph.org.uk – 189990.jpg”. Jun 21, 2006. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Severnside_fertilizer_works_-_geograph.org.uk_-_189990.jpg
[…] Leidus, Ivar. “File:Bombus rupestris – Trifolium pratense – Keila.jpg”. Jul 9, 2016. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Bombus_rupestris_-_Trifolium_pratense_-_Keila.jpg
[…] Vanek, Steve. “Figure 5.2.3”. Karsten, H. Vanek, S. Zimmerer, K. Gigi, R. Blumler, M. The Nitrogen Cycle and Human Management of Soils. Module 5.2. Food and the Future Environment. The College of Earth and Mineral Sciences. Pennsyvania State Univesity. Accessed Dec 19, 2024. https://www.e-education.psu.edu/geog3/node/1175
[…] Schmaltz, Jeff. “File:Sediment in the Gulf of Mexico (2).jpg”. Nov 13, 2009. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Sediment_in_the_Gulf_of_Mexico_(2).jpg
[…] Betts, Lynn. “File:NRCSIA99213 – Iowa (3121)(NRCS Photo Gallery).jpg”. Oct 4, 2011. USDA NCRS. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:NRCSIA99213(16876)(NRCS_Photo_Gallery).jpg
[…] Rosser1954. “File:Alder (Alnus glutinosa) tree with root nodules, Chapeltoun, North Ayrshire.jpg”. Mar 8, 2022. Wikimedia Commons. Accessed Mar 8, 2024. https://commons.wikimedia.org/wiki/File:Alder_(Alnus_glutinosa)_tree_with_root_nodules,_Chapeltoun,_North_Ayrshire.jpg
[62a] Garcia, Joshua. Kao-Kniffin, Jenny. “File:Enrichment of microbial groups in plant rhizospheres.jpg”. Jul 11, 2018. Wikimedia Commons. Accessed Dec 16, 2023. https://commons.wikimedia.org/wiki/File:Enrichment_of_microbial_groups_in_plant_rhizospheres.jpg
[63a] Jochen, Gschnaller. “File:PhosphComby.jpg”. Jul 11, 2009. Wikimedia Commons. Accessed Jan 8, 2025. https://commons.wikimedia.org/wiki/File:PhosphComby.jpg
[64a] Palmer, Alfred T. “File:Smoke stack of TVA chemical plant where elemental phosphorus is made1a35277v.jpg”. June 1942. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Smoke_stack_of_TVA_chemical_plant_where_elemental_phosphorus_is_made1a35277v.jpg
[…] Parker-Burlingham, Jason. “File:Phosphate Mine Panorama.jpg”. Jun 30, 2008. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Phosphate_Mine_Panorama.jpg
[…] عمروبن كلثوم. “File:نقص الفسفور على الذرة.jpg”. Jun 21, 2009. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:%D9%86%D9%82%D8%B5_%D8%A7%D9%84%D9%81%D8%B3%D9%81%D9%88%D8%B1_%D8%B9%D9%84%D9%89_%D8%A7%D9%84%D8%B0%D8%B1%D8%A9.jpg
[…] Vanek, Steven. “Figure 5.2.4”. Karsten, H. Vanek, S. Zimmerer, K. Gigi, R. Blumler, M. The Phosphorus Cycle and Human Management of Soils. Module 5.2. Food and the Future Environment. The College of Earth and Mineral Sciences.Pennsylvania State University. Accessed Mar 5, 2024. https://www.e-education.psu.edu/geog3/node/1176
[…] Marathon, Mark. “File:Bull chewing bone 2.jpg”. Jun 2, 2017. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Bull_chewing_bone_2.jpg
[…|65a] Selosse, Marc-André. “File:Ectomycorhizae of Scleroderma on Pinus (China).png”. May 29, 2020. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Ectomycorhizae_of_Scleroderma_on_Pinus_(China).png
[65a] Kottke, Ingrid. “File:ECM Schnitte.tif”. Feb 25, 2018. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:ECM_Schnitte.tif
[66a] Dnn87. “File:Potassium.JPG”. Dec 11, 2007. Wikimedia Commons. Accessed Mar 15, 2024. https://commons.wikimedia.org/wiki/File:Potassium.JPG
[67a] Seppel, Mai. “File:Estonian Museum of Natural History Specimen No 173341 photo (g1 g1-1623 1 jpg).jpg”. Mar 9, 2015. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Estonian_Museum_of_Natural_History_Specimen_No_173341_photo_(g1_g1-1623_1_jpg).jpg
[…] Ringwoodit. “File:Glaukonit XPL.jpg”. Mar 1, 2020. Wikimedia Commons. Accessed Mar 5, 2024. https://commons.wikimedia.org/wiki/File:Glaukonit_XPL.jpg
[…] Whalen, Joann.”Figure 7.11″. Whalen, JK. Ziadi, N. Schoenau, JJ. Paré, MC. Burton, DL. Bruulsema, T. No. 7. Soil Nutrient Cycling. Digging Into Canadian Soils. University of Saskatchewan. Accessed Mar 5, 2024. https://openpress.usask.ca/soilscience/chapter/soil-nutrient-cycling/
[68a] S. Sandlöbes et al. “File:Cold rolling of Mg and Mg-1Al-0.1Ca.jpg”. 2017. Wikimedia Commons. Accessed Apr 8, 2024. https://commons.wikimedia.org/wiki/File:Cold_rolling_of_Mg_and_Mg-1Al-0.1Ca.jpg
[…] Tobosha. “File:Emergency fire starter 20110709.jpg”. Jul 9, 2011. Wikimedia Commons. Accessed Apr 8, 2024. https://commons.wikimedia.org/wiki/File:Emergency_fire_starter_20110709.jpg
[…] Henle, Fritz. “File:Enormous asbestos mittens must be worn by men handling 8b08240v.jpg”. Jan 1943. Wikimedia Commons. Accessed Apr 8, 2024. https://commons.wikimedia.org/wiki/File:Enormous_asbestos_mittens_must_be_worn_by_men_handling_8b08240v.jpg
- Asbestos is a dangerous mineral which was popular for its ability to be turned into a pulp and then woven into thermal insulating fibers. Because of its properties, it easily falls apart and enters the air as sharp microscopic pieces, where it is inhaled into the lungs and damages the lung, similar to the effects of volcanic ash.
[69a] Nefronus. “File:Calcium sample.jpg”. Oct 12, 2020. Wikimedia Commons. Accessed Mar 15, 2024. https://commons.wikimedia.org/wiki/File:Calcium_sample.jpg
[70a] Jones, David. “File:Afton down.jpg”. May 9, 2009. Wikimedia Commons. Accessed Mar 15, 2024. https://commons.wikimedia.org/wiki/File:Afton_down.jpg
[…] Massagli, Andrea. “File:CRYSTALS OF TIME – CRISTALLI DI TEMPO.jpg”. Aug 18, 2017. Wikimedia Commons. Accessed Mar 15, 2024. https://commons.wikimedia.org/wiki/File:CRYSTALS_OF_TIME_-_CRISTALLI_DI_TEMPO.jpg
[…] Russty67. “File:Shells and sand By G,Russ.jpg”. Apr 2, 2012. Wikimedia Commons. Accessed Mar 15, 2024. https://commons.wikimedia.org/wiki/File:Shells_and_sand_By_G,Russ.jpg
[…] Monto, Tiia. “File:Cow skull.jpg”. May 12, 2018. Wikimedia Commons. Accessed Mar 19, 2024. https://commons.wikimedia.org/wiki/File:Cow_skull.jpg
[71a] El-Zaidy, Mohamed. Alsahli, Abdulaziz A. “Occurrence of calcium oxalate crystals in some wild plants used in traditional medicine in Saudi Arabia”. Feb 28, 2021. Academic Journals, vol. 15(2), pp. 96-107. https://academicjournals.org/journal/JMPR/article-full-text-pdf/316067A66153
[…] Konyar, ST. Öztürk, N. Dane, F. “Occurrence, types and distribution of calcium oxalate crystals in leaves and stems of some species of poisonous plants”. Mar 15, 2014. Botanical Studies, vol. 55, no. 32 (2014). https://doi.org/10.1186/1999-3110-55-32
[72a] Welcome1To1The1Jungle. “File:SulfurCycle copy.jpg”. May 15, 2014. Wikimedia Commons. Accessed Apr 8, 2024. https://commons.wikimedia.org/wiki/File:SulfurCycle_copy.jpg
[73a] Sharanbhurke. “File:Danakil.jpg”. Mar 17, 2019. Wikimedia Commons. Accessed Apr 8, 2024. https://commons.wikimedia.org/wiki/File:Danakil.jpg
[…] Leiem. “File:Chem Emoji Bad Smell.png”. Jun 1, 2019. Wikimedia Commons. Accessed Apr 8, 2024. https://commons.wikimedia.org/wiki/File:Chem_Emoji_Bad_Smell.png
[…] Magdawszedobylska. “File:Miners on Mount Ijen.jpg”. Oct 19, 2018. Wikimedia Commons. Accessed Apr 8, 2024. https://commons.wikimedia.org/wiki/File:Miners_on_Mount_Ijen.jpg
[74a] Sandbh. “File:Fertilizer.jpg”. Aug 12, 2016. Wikimedia Commons. Accessed Apr 2, 2024. https://commons.wikimedia.org/wiki/File:Fertilizer.jpg
[…] Mayes, Derek. “File:Cutting peat on Veness – geograph.org.uk – 2301177.jpg”. Jul 6, 2005. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Cutting_peat_on_Veness_-geograph.org.uk-_2301177.jpg
- Orkney Islands, Scotland, United Kingdom (UK)
[…] Jhatakiya, Hardik. “File:Hardik harshad and ketav.jpg”. Dec 22, 2020. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Hardik_harshad_and_ketav.jpg
- Urbanexotic, Ahmedabad, India
[…] Satin66Flower. “File:CutsProcona1ra.jpg”. Jun 17, 1999. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:CutsProcona1ra.jpg
[…] Alandmanson. “File:Soil fertility analysis 7 Samples for Zn Mn & Cu by AA.jpg”. Feb 8, 2012. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Soil_fertility_analysis_7_Samples_for_Zn_Mn_%26_Cu_by_AA.jpg
- Cedara College of Agriculture, Hilton, South Africa
[…] King, Franklin Hiram. “File:Farmers of forty centuries; or, Permanent agriculture in China, Korea and Japan (1900) (14799006843).jpg”. 1900. Farmers for forty centuries. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Farmers_of_forty_centuries;or,_Permanent_agriculture_in_China,_Korea_and_Japan(1900)_(14799006843).jpg
[75a] DanielCD. “File:Hematite.jpg”. Mar 13, 2005. Wikimedia Commons. Accessed Mar 28, 2024. https://commons.wikimedia.org/wiki/File:Hematite.jpg
[…] Motahari, Rezvan. “File:Gol Gohar iron ore mine 2019-10-23 12.jpg”. Oct 23, 2019. Wikimedia Commons. Accessed Mar 28, 2024. https://commons.wikimedia.org/wiki/File:Gol_Gohar_iron_ore_mine_2019-10-23_12.jpg
[…] Arnoldius. “File:Pelletized iron ore with scale.jpg”. Feb 13, 2010. Wikimedia Commons. Accessed Mar 28, 2024. https://commons.wikimedia.org/wiki/File:Pelletized_iron_ore_with_scale.jpg
[…] Motahari, Rezvan. “File:Gol Gohar iron ore mine 2019-10-23 23.jpg”. Oct 23, 2019. Wikimedia Commons. Accessed Mar 28, 2024. https://commons.wikimedia.org/wiki/File:Gol_Gohar_iron_ore_mine_2019-10-23_23.jpg
[…] Alchemist-hp. “File:Iron electrolytic and 1cm3 cube.jpg”. Apr 24, 2020. Wikimedia Commons. Accessed Mar 28, 2024. https://commons.wikimedia.org/wiki/File:Iron_electrolytic_and_1cm3_cube.jpg
[76a] Alandmanson. “File:2006 1017 Iron deficiency in peach.JPG”. Oct 17, 2006. Wikimedia Commons. Accessed Mar 28, 2024. https://commons.wikimedia.org/wiki/File:2006_1017_Iron_deficiency_in_peach.JPG
[…] Vincentz, Frank. “File:Banksia integrifolia 02 ies.jpg”. Jul 27, 2007. Wikimedia Commons. Accessed Mar 28, 2024. https://commons.wikimedia.org/wiki/File:Banksia_integrifolia_02_ies.jpg
[…] Eiku en exil. “File:Chlorose ferrique sur Citrus aurantium.jpg”. Mar 3, 2022. Wikimedia Commons. Accessed Mar 28, 2024. https://commons.wikimedia.org/wiki/File:Chlorose_ferrique_sur_Citrus_aurantium.jpg
[77a] Zander, Jonathan. “File:NatCopper.jpg”. Jul 6, 2009. Wikimedia Commons. Accessed Apr 2, 2024. https://commons.wikimedia.org/wiki/File:NatCopper.jpg
[…] Grobe, Hannes. “File:Malachit sophie-schulenburg hg.jpg”. Aug 5, 2010. Wikimedia Commons. Accessed Apr 2, 2024. https://commons.wikimedia.org/wiki/File:Malachit_sophie-schulenburg_hg.jpg
[…] Jahn, Reinhard. “File:Chuqui001.jpg”. Wikimedia Commons. Accessed Apr 2, 2024. https://commons.wikimedia.org/wiki/File:Chuqui001.jpg
[78a] Dnn87. “File:Na (Sodium).jpg”. Dec 12, 2007. Wikimedia Commons. Accessed Mar 15, 2024. https://commons.wikimedia.org/wiki/File:Na_(Sodium).jpg
[79a] Colsu. “File:Boutdeville – Langueux – Prés salés.jpg”. Feb 22, 2018. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Boutdeville_-_Langueux_-_Pr%C3%A9s_sal%C3%A9s.jpg
[…] Sauber, Wolfgang. “File:Bolsa Chica – Salzmarschen.jpg”. Apr 9, 2008. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Bolsa_Chica_-_Salzmarschen.jpg
[…] de Pedro, Raquel Sánchez. “File:Atriplex portulacoides.JPG”. Jun 12, 2014. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Atriplex_portulacoides.JPG
[…] Colsu. “File:Aster maritime (Tripolium pannonicum) dans les prés salés.jpg”. Oct 21, 2017. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Aster_maritime_(Tripolium_pannonicum)_dans_les_pr%C3%A9s_sal%C3%A9s.jpg
[…] Beck, Florent. “File:Plantago maritima fruit (03).jpg”. Sept 24, 2012. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Plantago_maritima_fruit_(03).jpg
- Saint-Jean-de-Luz, Pyrénées-Atlantiques, France
[80a] Stolbovsky. “File:Yaroslavich.jpg”. Mar 3, 2021. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Yaroslavich.jpg
[…] Roulex 45. “File:Disque d’épandage 2.JPG”. Feb 13, 2008. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Disque_d%27%C3%A9pandage_2.JPG
[…] “File:Balance del Plan de Emergencias Invernales de la ciudad de Madrid 02.jpg”. Feb 5, 2018. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Balance_del_Plan_de_Emergencias_Invernales_de_la_ciudad_de_Madrid_02.jpg
[…] “File:MTA Crews Respond to Winter Storm (50730205161).jpg”. Dec 17, 2020. Metropolitan Transportation Authority of the State of New York. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:MTA_Crews_Respond_to_Winter_Storm_(50730205161).jpg
[…] Covey, Al. “4310922760_85b9c21616_o”. Virginia Department of Transportation (VDOT). Flickr. Accessed Apr 4, 2024. https://www.flickr.com/photos/vadot/4310922760/in/photostream/
[81a] “File:Organico argentina.png”. Dec 31, 2001. Ministerio de Agricultura, Ganadería y Pesca. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Organico_argentina.png
[…] “File:Nasaa logo2.gif”. Feb 26, 2015. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Nasaa_logo2.gif
[…] “File:Bio-Siegel-EG-Öko-VO-Deutschland.svg”. Feb 06, 2002. Bundesministerium für Verbraucherschutz, Ernährung und Landwirtschaft. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Bio-Siegel-EG-%C3%96ko-VO-Deutschland.svg
[…] “File:Selo do SisOrg – MAPA.jpg”. Aug 22, 2011. Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Selo_do_SisOrg_-_MAPA.jpg
[…] “File:Canadian Organic Seal.png”. Aug 28, 2017. Canadian Food Inspection Agency. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Canadian_Organic_Seal.png
[…] “File:Kilimohai Organic.png”. Jan 4, 2014.Kenya Organic Agriculture Network. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Kilimohai_Organic.png
[…] “File:USDA organic seal.svg”. USDA. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:USDA_organic_seal.svg
[…] Kurbiko, Andrew J. “File:Organic certification label of Ukraine.svg”. Feb 16, 2015. Ministry of Health Ukraine. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Organic_certification_label_of_Ukraine.svg
[…] “File:Uniform Organic Logo.jpg”. Sept 10, 2008. Israel Ministry of Agriculture and Rural Development. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Uniform_Organic_Logo.jpg
[82a] Leidus, Ivar. “File:Apis mellifera – Senecio paludosus – Keila.jpg”. Jul 24, 2016. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Apis_mellifera_-Senecio_paludosus-_Keila.jpg
[…] Gallagher, Judy. “File:Green Sweat Bee – Augochlorella species, Mason Neck, Virginia (38251317511).jpg”. Oct 3, 2017. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Green_Sweat_Bee_-_Augochlorella_species,_Mason_Neck,_Virginia_(38251317511).jpg
[…] Nivis, Flocci. “File:20180708 Bombus 04.jpg”. Jul 8, 2018. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:20180708_Bombus_04.jpg
[…] “File:Imidacloprid USA 2011.png”. Mar 4, 2014. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Imidacloprid_USA_2011.png
[…] NEUROtiker. “File:Imidacloprid.svg”. Jan 13, 2008. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Imidacloprid.svg
[…] “File:2013-06-07 20-55-16-syrphidae.JPG”. Jun 7, 2013. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:2013-06-07_20-55-16-syrphidae.JPG
[…] Becks. “File:Two Flies (7080957493).jpg”. Apr 15, 2022. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Two_Flies_(7080957493).jpg
[…] Petts, James. “File:Flowers (14747543339).jpg”. Jul 27, 2014. Wikimedia Commons. Accessed Apr 4, 2024. https://commons.wikimedia.org/wiki/File:Flowers_(14747543339).jpg
[83a] Thompsma. “File:TrophicWeb.jpg”. May 31, 2011. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:TrophicWeb.jpg
[…] Gordy, Wilbur F. “File:Theiroquoislonghouse.png”. 1913. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Theiroquoislonghouse.png
[…] Wild, Siera. “File:Recycling sign green 2.png”. Jan 22, 2022. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Recycling_sign_green_2.png
[84a] Stas1995. “File:Two pieces of lead, 11 grams, 1 x 1.5 cm each.jpg”. Dec 15, 2019. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Two_pieces_of_lead,_11_grams,_1_x_1.5_cm_each.jpg
[…] Lavinsky, Robert M. “File:Calcite-Galena-t06-156b.jpg”. ~March 2010. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Calcite-Galena-t06-156b.jpg
[…] Kamenz, Rainer. “File:Aufbau Autobatterie.jpg”. Sep 8, 2020. Wikimedia Commons. Accessed Dec 19, 2024. https://commons.wikimedia.org/wiki/File:Aufbau_Autobatterie.jpg
[…] Cumpston, Mike. “File:Minie Balls.jpg”. March, 2008. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Minie_Balls.jpg
[…] Raboe001. “File:Angeln zubehoer grundblei 01.jpg”. Dec 10, 2005. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Angeln_zubehoer_grundblei_01.jpg
[…] Mangl, Odřej. “File:Oxid olovnatý.PNG”. June 24, 2007. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Oxid_olovnat%C3%BD.PNG
[…] Lamiot. “File:Peinture plomb écailles 3.jpg”. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Peinture_plomb_%C3%A9cailles_3.jpg
- house built in 1948
[85a] Viinamakelainen. “File:Decrease of dioxins in ambient air over 20 years.jpg”. Aug 5, 2019. Wikimedia Commons. Accessed July 2, 2024. https://commons.wikimedia.org/wiki/File:Decrease_of_dioxins_in_ambient_air_over_20_years.jpg
[…] Edgar181. “File:PCDD general structure.png”. Dec 9, 2008. Wikimedia Commons. Accessed July 2, 2024. https://commons.wikimedia.org/wiki/File:PCDD_general_structure.png
[…] Nyberg, Richard. “File:Site construction at the Environmental Remediation of Dioxin Contamination at Danang Airport (8681129258).jpg”. Apr 23, 2013. US AID Vietnam. Wikimedia Commons. Accessed July 2, 2024. https://commons.wikimedia.org/wiki/File:Site_construction_at_the_Environmental_Remediation_of_Dioxin_Contamination_at_Danang_Airport_(8681129258).jpg
[86a] Jshamgochian. “File:Black-capped Chickadee (Poecile atricapillus) excavating a nest cavity in Waterville, Maine.jpg”. Apr 25, 2022. Wikimedia Commons. Accessed Dec 19, 2024. https://commons.wikimedia.org/wiki/File:Black-capped_Chickadee_(Poecile_atricapillus)_excavating_a_nest_cavity_in_Waterville,_Maine.jpg
[…] Syced. “File:Taiko stage after Futako-Tamagawa fireworks 4.jpg”. Oct 21, 2023. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Taiko_stage_after_Futako-Tamagawa_fireworks_4.jpg
[…] Ngumenawe. “File:Group of dancers from Bamasaba.JPG”. Oct 14, 2011. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Group_of_dancers_from_Bamasaba.JPG
[…] Basile, Ricardo. “File:Orchestra della Toscana.jpg”. Jan 10, 2024. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Orchestra_della_Toscana.jpg
[…] Khaire, SS. “File:Group dance.jpg”. March 7, 2020. Wikimedia Commons. Accessed June 28, 2024. https://commons.wikimedia.org/wiki/File:Group_dance.jpg
[…] “File:20160628-OSEC-LSC-0219 (28114326995).jpg” Jun 28, 2024. USDA. Wikimedia Commons. Accessed Dec 19, 2024. https://commons.wikimedia.org/wiki/File:20160628-OSEC-LSC-0219_(28114326995).jpg

